Abstract
Background
Sinonasal bitter taste receptors (T2Rs) contribute to upper airway innate immunity and correlate with chronic rhinosinusitis (CRS) clinical outcomes. A subset of T2Rs expressed on sinonasal solitary chemosensory cells (SCCs) are activated by denatonium, resulting in a calcium-mediated secretion of bactericidal antimicrobial peptides (AMPs) in neighboring ciliated epithelial cells. We hypothesized that there is patient variability in the amount of bacterial killing induced by different concentrations of denatonium and that the differences correlate with CRS clinical outcomes.
Methods
Bacterial growth inhibition was quantified after mixing bacteria with airway surface liquid (ASL) collected from denatonium-stimulated sinonasal air-liquid interface (ALI) cultures. Patient ASL bacterial killing at 0.1 mM denatonium and baseline characteristics and sinus surgery outcomes were compared between these populations.
Results
There is variability in the degree of denatonium-induced bacterial killing between patients. In CRS with nasal polyps (CRSwNP), patients with increased bacterial killing after stimulation with low levels of denatonium undergo significantly more functional endoscopic sinus surgeries (FESSs) (p = 0.037) and have worse 6-month post-FESS 22-item Sino-Nasal Outcome Test (SNOT-22) scores (p = 0.012).
Conclusion
Bacterial killing after stimulation with low levels of denatonium correlates with number of prior FESS and postoperative SNOT-22 scores in CRSwNP. Some symptoms of CRS in patients with hyperresponsiveness to low levels of denatonium may be due to increased airway immune activity or inherent disease severity.
Keywords: sinusitis, FESS, SNOT-22, sinus surgery, taste receptors, denatonium
Patients with chronic rhinosinusitis (CRS) are often subdivided into the phenotypic categories CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP). At the cellular level, in white patients, CRSsNP is mainly T helper 1 (Th1)-mediated with elevated interferon-gamma (INF-γ) and interleukin 8 (IL-8) levels; while CRSwNP is Th2-mediated with increased IL-5, eosinophils, and mast cells.1 Although CRS categorizations are being further refined into inflammatory endotypes based on specific cytokines and cell types, these phenotypic independent molecular endotypes indeed “mirror clinical phenotypes, supporting their clinical relevance.”2
Taste receptors have been studied for their role in sinonasal immunity and contributions to CRS.3–10 The bitter taste receptor T2R38 in ciliated sinonasal cells is activated by gram-negative bacteria, resulting in bactericidal levels of nitric oxide (NO) production.11 A different upper airway cell type, the solitary chemosensory cell (SCC), has both bitter (T2R) and sweet (T1R) receptors. T2Rs in SCCs are activated by the bitter agonist denatonium benzoate (denatonium), leading to a calcium wave that induces secretion of bactericidal antimicrobial peptides (AMPs), including β-defensins 1 and 2. Notably, when the sweet receptor T1R2/3 in SCCs is activated by apical sugars it suppresses the SCC T2R-mediated AMP secretion triggered by denatonium.12
While polymorphisms in T2R38 have been correlated to gram-negative sinus infections,11 the presence of culturable bacteria colonizing the sinuses,10 and disease severity in CRSsNP,4–6 there are no studies investigating the clinical effects of the SCC T2R and T1R system activated by denatonium. We hypothesized that there is patient variability in bacterial killing induced by different concentrations of denatonium, and differences in killing correlate with clinical symptoms and sinus surgery outcomes. To test our hypotheses, we compared in vitro denatonium-induced sinonasal bacterial killing to clinical outcomes in CRS patients undergoing functional endoscopic sinus surgery (FESS).
Materials and methods
Sinonasal air-liquid interface cultures
Cultures were prepared as described.11,13 Briefly, surgical specimens of sinonasal mucosa were obtained from adults (age > 18 years) who met the Academy of Otolaryngology-Head and Neck Surgery clinical practice guidelines for adult CRS,14 had failed medical therapy, and were undergoing functional endoscopic sinus surgery (FESS) at the Department of Otorhinolaryngology at the University of Pennsylvania and the Philadelphia Veterans Affairs Medical Center. The Institutional Review Boards at both centers provided full study approval and informed consent was obtained preoperatively from all patients. Exclusion criteria included history of systemic diseases such as granulomatosis with polyangiitis, sarcoidosis, cystic fibrosis, immunodeficiency syndromes, or prescriptions for oral corticosteroids, antibiotics, or biologics (eg, Xolair) within the month preceding surgery. Air-liquid interface (ALI) cultures were prepared by enzymatically dissociating the epithelial cells from the sinonasal tissue and growing them to confluence in tissue culture flasks (75 cm2) using bronchial epithelial basal medium (BEBM; Clonetics, Cambrex, East, NJ) and proliferation medium consisting of Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F-12 media with 100 µg/mL streptomycin and 100 U/mL penicillin for 7 days. The epithelial cells were treated with trypsin and seeded on porous polyester membranes (67 × 104 cells per membrane) in cell culture inserts (Transwell-clear, 12-mm diameter, 0.4-µm pores; Corning, Acton, MA) coated with 100 µL of coating solution (bovine serum albumin [BSA; 0.1 mg/mL; Sigma-Aldrich, St. Louis, MO] and fibronectin [10 µg/mL; BD Biosciences, San Jose, CA] in Lechner and LaVeck [LHC] basal medium [Invitrogen, Grand Island, NY]) and bovine type I collagen (30 µg/mL; BD Biosciences). The cell cultures were placed in a tissue culture laminar flow hood for 12 hours. The culture media was removed from the apical side after 5 days and the epithelium was differentiated using medium consisting of 1:1 DMEM (Invitrogen) and bronchial epithelial basal medium (BEBM) (Clonetics Airways Epithelial Cell Systems; Cambrex Bio Science, Walkersville Inc., MD) and with the Clonetics complements for bovine pituitary extract (BPE) (0.13 mg/mL), human epidermal growth factor (hEGF) (0.5 ng/mL), insulin (5 g/mL), hydrocortisone (0.5 g/mL), triiodothyronine (6.5 g/mL), epinephrine (5 g/mL), and transferrin (0.5 g/mL), supplemented with 100 g/mL streptomycin, 100 U/mL penicillin, 0.1 nM retinoic acid (Sigma-Aldrich), and 10% fetal bovine serum (FBS) (Sigma-Aldrich) in the basolateral side. All experiments were performed on ALIs with visible cilia at 4 to 10 weeks after transitioning to the airliquid phase.
Bacteria culture and bacterial kill assays
Pseudomonas aeruginosa (strain PAO-1) was grown overnight at 37°C with shaking in lysogeny broth (LB) medium. Twenty-four hours prior to all kill assays, sinonasal ALI cultures were washed 4 times with Dulbecco’s phosphate-buffered saline (PBS) on the apical and basolateral surfaces. The apical surface was aspirated dry and the cultures were placed in antibiotic-free, serum-free media (50% DMEM, 50% BEBM; containing no antimicrobial agents) on the basolateral side. Two hours before the assay, the antibiotic-free, serum-free basolateral media was changed, and the apical side was washed 4 times with 300 µL PBS. Cultures were then overlaid with either 30 µL of 50% PBS or denatonium benzoate (Sigma-Aldrich) at 0.1, 1.0, or 10.0mMin 50% PBS. After the ALIs were incubated for 30 minutes at 37°C, the apical surface liquid (ASL) was removed and 20 µL ASL was mixed with 20 µL log-phase bacteria (grown overnight in 100% media, diluted to 0.0001 in 25% media, and incubated for 1 hour at 37°C with shaking) in 1 well of a 96-well plate. After shaking on a titer plate shaker for 1 minute at 1000 rpm the plates were incubated for 2 hours at 37°C with 15 seconds of shaking on the titer plate shaker every 30 minutes. Ten-fold serial dilutions were made using 25% bacterial media (1 × 100, 1 × 10−1, 1 × 10−2, 1 × 10−3 colony-forming units [CFUs]) and spotted on LB plates using a multichannel pipette. CFUs were counted using the dilution with approximately 10 to 30 colonies present; if no colonies were observed, the CFUs were scored as 0. To control for the variability in the number of raw CFUs in each experiment, results were normalized to the CFUs recovered when ASL from unstimulated cultures (PBS only) from the same patients were mixed with bacteria.
Study population and data collection
All surgeries were performed by 4 fellowship-trained rhinologists. Preoperative evaluations were conducted by rhinologists or trained researchers through in-person interviews, physical examinations, and a chart review. Coexisting medical conditions were self-reported by patients. Preoperative nasal endoscopy was performed by the rhinologists to determine nasal polyp status. Lund-Mackay scores were quantified by preoperative computed tomography (CT) scans.15 The degree of surgery performed was based on the surgeon’s judgment.
The study only incorporated patients who had outcomes scores available at 3, 6, and/or 12 months postoperatively as well as denatonium-induced bacterial kill assay data. Patients were analyzed based on the responsiveness of their cells to 0.1 mM denatonium, the lowest concentration tested in the bacterial kill assays.
Outcome variables
Baseline characteristics and sinonasal clinical outcomes were compared between hyperresponders and responders. The 22-item Sino-Nasal Outcome Test (SNOT-22), a validated quality of life survey of the severity of nasal disease symptoms experienced by patients over the preceding 2 weeks,16 was compared to measure subjective improvement in sinonasal symptoms after FESS. This symptom index was investigated because improvement in SNOT-22 scores postoperatively have been shown to correlate with long-term success after FESS.17
Statistical analysis
GraphPad Prism (GraphPad Software, Inc., La Jolla, CA) and Stata 13 (Stata Statistical Software, College Station, TX) were used for statistical analysis, with p < 0.05 considered statistically significant. Linear regression was used to define and analyze the relationship of baseline characteristics with bacterial inhibition at 0.1 mM denatonium as a continuous variable. All available SNOT-22 scores were compared at each time point. To compare SNOT-22 outcomes with denatonium sensitivity, a mixed model was created using bacterial inhibition at 0.1 mM denatonium as a predictor. Data are reported as means ± standard deviation (SD); “*” indicates p < 0.05.
Results
There is patient variability in denatonium-induced bacterial killing
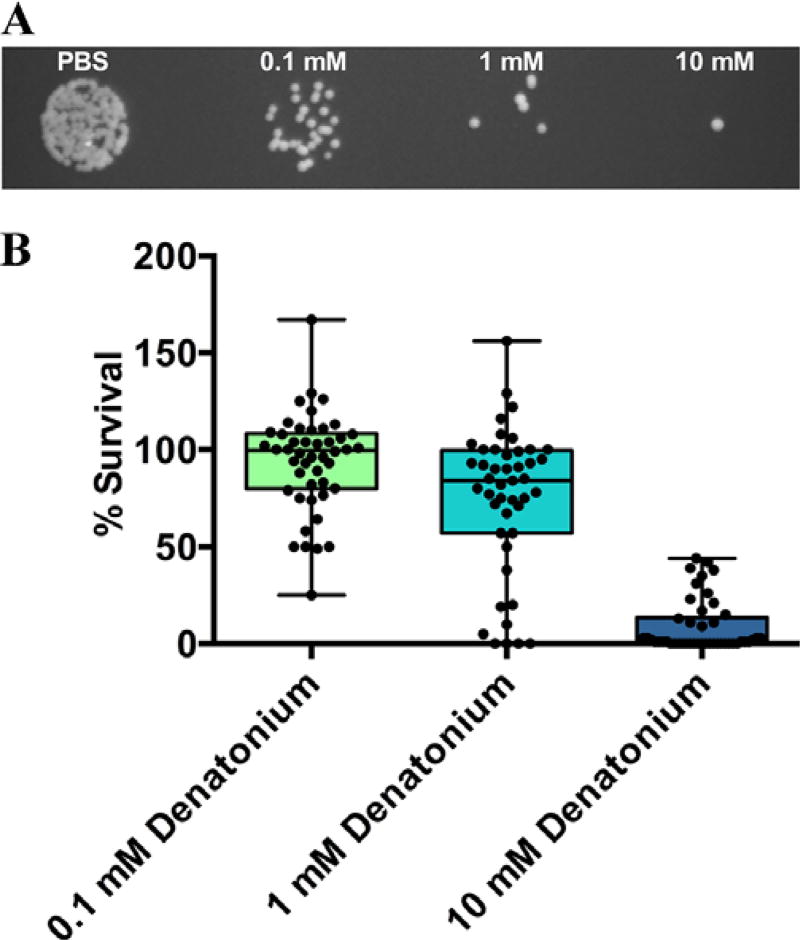
After stimulating the apical side of human sinonasal ALI cultures with PBS in the presence of different concentrations of denatonium for 30 minutes at 37°C and collecting and mixing the ASL with Pseudomonas aeruginosa, we observed that ASL from denatonium-stimulated cultures had variable bactericidal effects between patients (Fig. 1A). After normalizing to CFUs after stimulation with PBS alone, the average percentage CFUs remaining after stimulation with 0.1, 1.0, and 10.0 mM denatonium were 93.84 ± 25.31, 76.98 ± 41.67, and 8.652 ± 13.43, respectively (Fig. 1B).
FIGURE 1.
Patient variability in denatonium-induced Pseudomonas killing. (A) Example plate with Pseudomonas CFUs when bacteria were mixed with ASL from ALI cultures stimulated with PBS, 0.1, 1.0, and 10.0 mM denatonium. (B) Pseudomonas percentage survival after bacteria were mixed with ASL from ALIs stimulated with different concentrations of denatonium (normalized to number of CFUs after PBS stimulation for each individual culture). Average percentage survival after stimulation with 0.1, 1.0, and 10.0 mM denatonium were 93.84 ± 25.31, 76.98 ± 41.67, and 8.652 ± 13.43, respectively (n = 46 patients). Box and whiskers plot shows mean ± SD (box) with whiskers from minimum to maximum and circles indicating individual patient percentage survivals. ALI = air-liquid interface; ASL = airway surface liquid; CFUs = colony forming units; SD = standard deviation.
Patients with increased responsiveness to denatonium have significantly more FESS
There were a total of 46 patients with both denatonium-induced bacterial kill assays performed and clinical outcomes available between 3 and 12 months postoperatively. The effects of baseline characteristics on bacterial inhibition at 0.1 mM denatonium was assessed (Table 1). Prior FESS surgeries were associated with increased denatonium responsiveness (9% increased bacterial inhibition per prior FESS surgery; p = 0.037). Lund-Mackay scores were not significantly different based on responsiveness (0.86% inhibition per point, p = 0.43).Other baseline characteristics, including polyp status, were not significantly different. Of note, preoperative SNOT-22 scores were not significantly different between CRSwNP and CRSsNP patients. However, CRSwNP patients had significantly greater propensity for asthma and aspirin sensitivity, as expected.18
TABLE 1.
Relationship of preoperative characteristics of patients and response to denatonium*
| Predictors | Coefficient (change in % inhibition) |
p |
|---|---|---|
| Gender (male) | 9.8 | 0.53 |
| Age (each year) | 0 | 0.92 |
| Ethnicity (white)a | −3.5 | 0.85 |
| BMI (each point) | −1.0 | 0.23 |
| Smoking status | 14.6 | 0.33 |
| Polyps | −15 | 0.34 |
| Asthma | 13.2 | 0.32 |
| Diabetes | 3.3 | 0.84 |
| Aspirin sensitivity | 11.0 | 0.40 |
| Allergic rhinitis | 7.9 | 0.54 |
| Allergic fungal sinusitis | 2.1 | 0.91 |
| Prior FESS (n) | 9.0 | 0.037 |
| Lund-Mackay score | 0.9 | 0.43 |
| Preoperative SNOT-22 score | −0.1 | 0.74 |
The relationship of several baseline characteristics to patient cell culture response to 0.1 mM denatonium was analyzed. There were no significant effects of baseline characteristics with the exception of number of prior FESS surgeries (9.0% increased inhibition per FESS surgery, p = 0.037, linear regression model) (values in bold are significant at p < 0.05); table includes coefficient of regression model (as change in % inhibition).
Ethnicity is self-reported.
FESS = functional endoscopic sinus surgery; SNOT-22 = 22-item Sino-Nasal Outcome Test.
Denatonium hyperresponders have worse surgical outcomes in CRSwNP
Patients were classified based on the presence or absence of polyps on preoperative nasal endoscopy (CRSwNP n = 28; CRSsNP n = 17). Preoperative SNOT-22 scores were not significantly different between CRSwNP and CRSsNP groups, but were significantly lower in CRSwNP patients at 3 months (14.6 ± 14.8 vs 33.1 ± 14.6) and 6 months (15.9 ± 17.7 vs 34.3 ± 13.3) postoperatively (p < 0.05).
Postoperative SNOT-22 and total antibiotic and steroid courses during the 12 months postoperation were assessed for relationships with patient response to denatonium within the CRSwNP and CRSsNP groups. In the CRSwNP group, SNOT-22 scores were significantly higher 6 months postoperatively in individuals whose cells were more responsive to denatonium stimulation (mixed model coefficient 0.30, p = 0.012) (Table 2). While a similar effect was observed at other postoperative SNOT-22 assessments, this did not reach significance at 3 months (p = 0.16) and 12 months (p = 0.20). In this group, neither total postoperative antibiotic courses (coefficient −6.8, p = 0.21) nor total steroid courses (coefficient 3.1, p = 0.60) were strongly associated with responsiveness to denatonium. In the CRSsNP group, SNOT-22 scores and total postoperative steroid or antibiotic usage did not segregate by denatonium responsiveness to any appreciable degree (Table 2).
TABLE 2.
Relationship of postoperative outcomes and response to denatonium divided by polyp status*
| Predictors | Coefficient (change in % inhibition) |
p |
|---|---|---|
| CRSwNP (n = 28) | ||
| SNOT-22 | ||
| 3 months | 0.19 | 0.16 |
| 6 months | 0.30 | 0.012 |
| 12 months | 0.18 | 0.20 |
| Antibiotics | −6.9 | 0.21 |
| Steroids | 3.1 | 0.60 |
| CRSsNP (n = 17) | ||
| SNOT-22 | ||
| 3 months | −0.058 | 0.44 |
| 6 months | 0.069 | 0.96 |
| 12 months | 0.012 | 0.97 |
| Antibiotics | −0.33 | 0.96 |
| Steroids | −5.3 | 0.46 |
Patients were classified based on the presence (n = 28) or absence of nasal polyps (n = 17). The relationship of postoperative SNOT-22 scores and antibiotic and steroid courses to patient cell culture response to 0.1 mM denatonium was analyzed. In CRSwNP, SNOT-22 scores were significantly higher 6 months postoperatively in individuals whose cells were more responsive to denatonium stimulation (mixed model coefficient 0.30, p = 0.012) (values in bold are significant at p < 0.05); similarly, there was a trend toward significance at 3 months (0.19, p = 0.16) and 12 months (0.18, p = 0.20). All other outcomes listed were not significant. Table includes coefficient of regression model (as change in % inhibition).
CRSsNP = CRS without nasal polyps; CRSwNP = CRS with nasal polyps; SNOT-22 = 22-item Sino-Nasal Outcome Test.
Discussion
This is the first study demonstrating (1) patient variability in denatonium-induced bacterial killing by differentiated sinonasal epithelial cells and (2) a correlation between clinical outcomes and elevated bacterial killing at low levels of denatonium stimulation (0.1 mM) in vitro. These data may support previous work suggesting that T2Rs have roles in sinonasal innate immunity.3, 11,19 Importantly, previous translational work focused on T2R38 expressed on ciliated epithelial cells and its role in CRSsNP; this study is the first to highlight the fact that T2Rs expressed on a distinctly different cell population, SCCs, which do not express T2R38, may have a role in CRSwNP.
We previously demonstrated that CRS patients had elevated glucose in the ASL which led to T1R-mediated suppression of the SCC T2R AMP-secretion pathway.12 Here we describe possible clinical implications of the opposite end of the spectrum for SCC T2R immune defense—the effects of hyperresponsiveness, rather than blunted activity from increased inhibition. We found that individuals with an increased response to denatonium tend to have more prior FESS. These data may suggest that patients with hypersensitivity in SCC T2R-mediated AMP secretion have CRS pathology partially due to an overactive inflammatory environment. Specifically, elevated airway AMPs, such as β-defensins, or bacterial remnants could possibly be a contributory factor in airway damage20 and inflammation in some individuals. In support of this theory, AMPs are known to activate various airway cell types, including epithelial cells and mast cells, and have a role in regulation of cytokine production, cell differentiation, proliferation, and migration; moreover, shifts in the level of AMPs are associated with development of allergic diseases including allergic rhinitis, asthma, and CRS.21
Importantly, because this study is purely correlative, it is entirely possible that the multiple surgeries and refractory nature of disease in the hyperresponsive patients actually led to the increased responsiveness to denatonium rather than serving as a downstream consequence of hyperresponsiveness. The differences in denatonium-induced bacterial killing between patients also may be due to genetic differences, highlighting the importance of further studies investigating specific denatonium-activated SCC T2Rs. Alternatively, this data could reflect a higher density of SCC in the hyperresponsive patients and cultures, although 1 prior study did not find differences in SCCs between CRS and control subjects.22
Notably, prior heterologous expression experiments utilizing HEK-293 cells have demonstrated that denatonium activates multiple bitter taste receptors, including T2R4, 8, 10, 16, 39, 43, 46, and 47,23 but the physiologic effects of denatonium in the sinonasal epithelium (both in primary cultures and explants) appears to localize to nonciliated SCCs.12 This represents somewhat of a paradox, especially since some of the denatonium responsive T2Rs (T2R4 and T2R16) have recently been demonstrated to be expressed in ciliated cells.24 A possible explanation for this apparent contradiction may rest in the fact that the HEK-293 cells—though useful in studying the basic science of T2R receptors—cannot be expected to fully recapitulate a receptor in vivo, where hetero-oligomerization (both with other T2Rs and other families of G-protein-coupled receptors [GPCRs], as demonstrated by the recent work by Kim et al.25), biased agonism, and other factors may play an important role. Furthermore, the HEK-293 expression system utilizes receptors that are artificially tagged (rat SST3 receptor at the N terminus) and forced to couple to an overexpressed chimeric G-protein. Any of these components could alter signal transduction compared with primary endogenously expressed T2Rs. Last, T2R4, even in vitro, is a relatively low-affinity receptor for denatonium (the half-maximal effective concentration [EC50] ~ 300 µM vs 3 µM for T2R10 and 0.03 µM for T2R30/47).23 It may be that the concentrations of denatonium used in this study are sufficient to activate T2R10 and T2R30 in SCCs (previously postulated to be in these cells12), but not T2R4 in ciliated cells.
In this study, there was no correlation between denatonium-responsiveness and postoperative SNOT-22 scores for CRSsNP patients; however, CRSwNP patients that were more responsive to low levels of denatonium had significantly worse SNOT-22 scores at 6 months post-FESS. The correlation in CRSwNP but not CRSsNP supports the argument that there are multiple CRS endotypes with distinct pathophysiologies.26 Importantly, prior work has demonstrated that quality-of-life outcomes at 6 months following surgery are predictive of long-term outcomes and do not change appreciably between 6, 12, and 20 months27; regardless, one would have expected that in this study, the 3-month and 12-month SNOT-22 scores for CRSwNP would also correlate with denatonium responsiveness, rather than only trending toward significance.
In regard to postoperative outcomes, this study focused on a subjective, symptom-based measure, the SNOT-22 score; antibiotic and steroid courses served as the only postoperative objective measures of disease and showed no correlation with denatonium-responsiveness in both CRSwNP and CRSsNP. There are various additional limitations of this study, including the small sample size, particularly after dividing the cohort by polyp status, and the limited duration of follow-up. The small sample size (n = 46) is at least partially attributed to the fact that the study required both ALI bacterial kill assay data and 3 to 12 months of clinical follow-up for each patient enrolled. Furthermore, the study was limited to patients with CRS necessitating surgical intervention and thus the findings may not be applicable to all types of CRS or milder forms. We attempted to control for possible confounding variables in our statistical analysis when possible, but clinical and surgical outcomes are inherently multifactorial.
There was no correlation between baseline Lund-Mackay scores and denatonium-responsiveness. Importantly, a number of prior studies have demonstrated a weak correlation between CRS disease symptoms and CT findings.28–30 Nevertheless, other work has shown that volume-based CT scoring correlates with symptom-burden.31 Our dataset did not have volumetric scoring available for analysis. No correlation was found between the number of prior FESS and SNOT-22 or Lund-Mackay scores. Moreover, a regression comparing prior FESS and baseline endoscopy was unable to be performed due to insufficient data available for statistical analysis.
Conclusion
There is variability in the degree of denatonium-induced bacterial killing between patients. Bacterial killing after stimulation with low levels of denatonium correlates with CRS outcomes. Specifically, patients who have increased responsiveness to low concentrations of denatonium have undergone increased number of prior FESS, and the subset of CRSwNP report worse 6-month postoperative SNOT-22 scores. CRS in patients hyperresponsive to denatonium may be related to inherent disease severity and hyperresponsiveness may be a marker of elevated immune reactivity and inflammation.
Acknowledgments
Funding sources for the study: USPHS grant R01DC013588 (to N.A.C.).
Footnotes
Potential conflict of interest: None provided.
References
- 1.Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–1289. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 2.Tomassen P, Vandeplas G, Van Zele T, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137:1449–1456.e4. doi: 10.1016/j.jaci.2015.12.1324. [DOI] [PubMed] [Google Scholar]
- 3.Carey RM, Lee RJ, Cohen NA. Taste receptors in upper airway immunity. Adv Otorhinolaryngol. 2016;79:91–102. doi: 10.1159/000445137. [DOI] [PubMed] [Google Scholar]
- 4.Adappa ND, Farquhar D, Palmer JN, et al. TAS2R38 genotype predicts surgical outcome in nonpolypoid chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6:25–33. doi: 10.1002/alr.21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adappa ND, Howland TJ, Palmer JN, et al. Genetics of the taste receptor T2R38 correlates with chronic rhinosinusitis necessitating surgical intervention. Int Forum Allergy Rhinol. 2013;3:184–187. doi: 10.1002/alr.21140. [DOI] [PubMed] [Google Scholar]
- 6.Adappa ND, Zhang Z, Palmer JN, et al. The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int Forum Allergy Rhinol. 2014;4:3–7. doi: 10.1002/alr.21253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mfuna Endam L, Filali-Mouhim A, Boisvert P, Boulet LP, Bosse Y, Desrosiers M. Genetic variations in taste receptors are associated with chronic rhinosinusitis: a replication study. Int Forum Allergy Rhinol. 2014;4:200–206. doi: 10.1002/alr.21275. [DOI] [PubMed] [Google Scholar]
- 8.Dzaman K, Zagor M, Sarnowska E, Krzeski A, Kantor I. The correlation of TAS2R38 gene variants with higher risk for chronic rhinosinusitis in Polish patients. Otolaryngol Pol. 2016;70:13–18. doi: 10.5604/00306657.1209438. [DOI] [PubMed] [Google Scholar]
- 9.Gallo S, Grossi S, Montrasio G, et al. TAS2R38 taste receptor gene and chronic rhinosinusitis: new data from an Italian population. BMC Med Genet. 2016;17:54. doi: 10.1186/s12881-016-0321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rom DI, Christensen JM, Alvarado R, Sacks R, Harvey RJ. The impact of bitter taste receptor genetics on culturable bacteria in chronic rhinosinusitis. Rhinology. 2017;55:90–94. doi: 10.4193/Rhin16.181. [DOI] [PubMed] [Google Scholar]
- 11.Lee RJ, Xiong G, Kofonow JM, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012;122:4145–4159. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee RJ, Kofonow JM, Rosen PL, et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest. 2014;124:1393–1405. doi: 10.1172/JCI72094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai Y, Chen B, Shi J, Palmer JN, Kennedy DW, Cohen NA. Inflammation-mediated upregulation of centrosomal protein 110, a negative modulator of ciliogenesis, in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2011;128:1207–1215.e1. doi: 10.1016/j.jaci.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137:S1–S31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 15.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31:183–184. [PubMed] [Google Scholar]
- 16.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34:447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy JL, Hubbard MA, Huyett P, Patrie JT, Borish L, Payne SC. Sino-nasal outcome test (SNOT-22): a predictor of postsurgical improvement in patients with chronic sinusitis. Ann Allergy Asthma Immunol. 2013;111:246–251.e2. doi: 10.1016/j.anai.2013.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.London NR, Jr, Reh DD. Differential diagnosis of chronic rhinosinusitis with nasal polyps. Adv Otorhinolaryngol. 2016;79:1–12. doi: 10.1159/000444957. [DOI] [PubMed] [Google Scholar]
- 19.Lee RJ, Cohen NA. Bitter and sweet taste receptors in the respiratory epithelium in health and disease. J Mol Med. 2014;92:1235–1244. doi: 10.1007/s00109-014-1222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chennupati SK, Chiu AG, Tamashiro E, et al. Effects of an LL-37-derived antimicrobial peptide in an animal model of biofilm Pseudomonas sinusitis. Am J Rhinol Allergy. 2009;23:46–51. doi: 10.2500/ajra.2009.23.3261. [DOI] [PubMed] [Google Scholar]
- 21.Niyonsaba F, Kiatsurayanon C, Ogawa H. The role of human beta-defensins in allergic diseases. Clin Exp Allergy. 2016;46:1522–1530. doi: 10.1111/cea.12843. [DOI] [PubMed] [Google Scholar]
- 22.Barham HP, Cooper SE, Anderson CB, et al. Solitary chemosensory cells and bitter taste receptor signaling in human sinonasal mucosa. Int Forum Allergy Rhinol. 2013;3:450–457. doi: 10.1002/alr.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyerhof W, Batram C, Kuhn C, et al. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 2010;35:157–170. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- 24.Yan CH, Hahn S, McMahon D, et al. Nitric oxide production is stimulated by bitter taste receptors ubiquitously expressed in the sinonasal cavity. Am J Rhinol Allergy. 2017;31:85–92. doi: 10.2500/ajra.2017.31.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D, Pauer SH, Yong HM, An SS, Liggett SB. beta2-Adrenergic receptors chaperone trapped bitter taste receptor 14 to the cell surface as a heterodimer and exert unidirectional desensitization of taste receptor function. J Biol Chem. 2016;291:17616–17628. doi: 10.1074/jbc.M116.722736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akdis CA, Bachert C, Cingi C, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2013;131:1479–1490. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soler ZM, Smith TL. Quality-of-life outcomes after endoscopic sinus surgery: how long is long enough? Otolaryngol Head Neck Surg. 2010;143:621–625. doi: 10.1016/j.otohns.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garneau J, Ramirez M, Armato SG, 3rd, et al. Computer-assisted staging of chronic rhinosinusitis correlates with symptoms. Int Forum Allergy Rhinol. 2015;5:637–642. doi: 10.1002/alr.21499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan WR, Ramachandra T, Hwang PH. Correlations between symptoms, nasal endoscopy, and in office computed tomography in post-surgical chronic rhinosinusitis patients. Laryngoscope. 2011;121:674–678. doi: 10.1002/lary.21394. [DOI] [PubMed] [Google Scholar]
- 30.Jones NS. CT of the paranasal sinuses: a review of the correlation with clinical, surgical and histopathological findings. Clin Otolaryngol Allied Sci. 2002;27:11–17. doi: 10.1046/j.0307-7772.2001.00525.x. [DOI] [PubMed] [Google Scholar]
- 31.Pallanch JF, Yu L, Delone D, et al. Three-dimensional volumetric computed tomographic scoring as an objective outcome measure for chronic rhinosinusitis: clinical correlations and comparison to Lund-Mackay scoring. Int Forum Allergy Rhinol. 2013;3:963–972. doi: 10.1002/alr.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]