Abstract
Background and Objectives
We defined laboratory marker profiles typical of incomplete Kawasaki disease (iKD) during illness, especially with respect to the presence of a coronary artery abnormality such as coronary artery dilation or aneurysm.
Methods
This retrospective study examined the clinical and laboratory markers of patients with iKD over time, along with those of patients with complete KD (cKD) and febrile controls.
Results
Of 795 patients, 178 had iKD, 504 had cKD and 113 were febrile controls. During the transition from the acute to subacute phase, the age-adjusted hemoglobin levels and platelet counts were significantly lower and higher, respectively, in the subacute phase than in the acute phase in both iKD and cKD patients, which differed from those of febrile controls. Lower levels of acute and subacute age-adjusted hemoglobin levels in iKD patients (odds ratio [OR], 0.538 and 0.583; p=0.006 and 0.018, respectively) and higher subacute platelet counts in cKD patients (OR, 1.004; p=0.014) were correlated with the risk of coronary dilation. A higher acute neutrophil-to-lymphocyte ratio was associated with aneurysm only in cKD patients (OR, 1.059; p=0.044).
Conclusions
The iKD patients share KD-specific laboratory marker profiles in terms of complete blood cell counts and acute phase reactant levels with cKD patients. However, the factors predicting coronary dilation differ according to the phenotype; lower acute and subacute age-adjusted hemoglobin levels predict coronary dilation only in iKD patients.
Keywords: Mucocutaneous lymph node syndrome, Coronary artery disease, Biomarkers
INTRODUCTION
Kawasaki disease (KD) is an acute febrile vasculitis syndrome involving medium-sized blood vessels and is most common in children. It is often complicated by coronary artery abnormalities (CAAs), and has become a leading cause of acquired heart disease worldwide.1) The etiology is unknown, but the disease is characterized by clinical manifestations and laboratory features typically associated with systemic inflammation. If clinical manifestations are not diagnostic, laboratory features commonly observed in those with complete KD (cKD) can be used to support the diagnosis of incomplete KD (iKD).1),2) However, it is not determined whether, unlike clinical manifestations, the laboratory features of iKD are complete during illness, compared with those of cKD. Considering that iKD is a known risk factor for the development of CAAs,3),4),5),6),7) the laboratory profiles of iKD need to be defined to assess the factors predicting CAAs.
Knowledge of the typical patterns of laboratory markers in KD over time is important, not only from a diagnostic point-of-view but also for estimating disease progression. Among the many laboratory markers associated with KD, complete blood cell counts/profiles and acute phase reactant levels change over time.8) For example, during the acute febrile phase of KD, most patients show both an increased white blood cell count with a predominance of neutrophils and elevated levels of acute phase reactants.1),8),9) During defervescence, neutrophilic leukocytosis tends to decrease. Platelet counts increase during the second week after disease onset, and return to normal 4 to 8 weeks after disease onset.1),8) Thus, laboratory markers change dynamically throughout the entire course of the disease. The careful monitoring of dynamic changes during periods of KD could assist in arriving at a correct diagnosis. In this study, we compared the dynamics of laboratory markers in iKD with those in cKD and other febrile diseases. Then, we assessed if there were any specific to iKD or cKD and also whether or not they correlated with the development of CAAs. Monitoring such changes could help in the assessment of iKD as a known risk factor for CAAs.
METHODS
Subjects
This retrospective cohort study reviewed the medical records of consecutive patients with KD who were treated with intravenous immunoglobulin (IVIG) at Korea University Medical Center between January 2008 and December 2014. Patients were classified as having cKD if they exhibited 4 or more of the principal clinical manifestations of KD, in addition to the typical fever associated with this disease.1) Patients were classified as having iKD after exclusion of other diagnoses if 2 or 3 manifestations of KD plus fever were supported by symptoms often associated with KD and by increased inflammatory indices.1) Of the patients with iKD, only those who exhibited typical late subacute manifestations, such as desquamation or thrombocytosis, were included in the analysis. Normal echocardiographic findings did not exclude a diagnosis of iKD. The study included only those patients who underwent laboratory analysis of complete blood cell counts, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) concentrations during the acute febrile phase before IVIG treatment and at 2 days (subacute phase) and 3–4 weeks (convalescent phase) after defervescence. The neutrophil-to-lymphocyte ratio (NLR) was calculated by dividing the neutrophil count by the lymphocyte count. The age-adjusted hemoglobin concentration was calculated using the following equation8),10):
Age-adjusted hemoglobin concentration=(observed hemoglobin level−mean hemoglobin level for age)/standard deviation for age
All patients were treated with IVIG (2 g/kg). Patients who were febrile more than 48 hours after IVIG completion were defined as IVIG-resistant, in which case they received a second dose of IVIG. Those continuing to show IVIG resistance were treated with intravenous methylprednisolone and an oral steroid. Echocardiograms were performed during the acute febrile phase and repeated 3–4 and 8–10 weeks later. A CAA was diagnosed according to the criteria of the Japanese Kawasaki Disease Research Committee11): a CAA at any time was considered, regardless of later resolution. The CAA group was further subdivided into 2 groups depending on whether it is diffuse ectatic or localized saccular: those with coronary artery dilation and those with an aneurysm. The febrile control group included patients less than 5 years of age who had a fever exceeding 38°C for more than 4 days plus at least one clinical criterion of KD during the same time period. Laboratory parameters of the cKD and iKD groups were compared with those of the febrile control group during the acute febrile phase and, if possible, 2 days after defervescence. This study was approved by Institutional Review Board of Korea University Guro Hospital (approval number: KUGH10229 ).
Statistical analysis
Statistical analyses were performed using SPSS 20 software (SPSS Inc., Chicago, IL, USA). Data were expressed as the median Q2 value (25th percentile Q1, 75th percentile Q3). Continuous variables were compared using the Mann-Whitney U test to estimate the significance of incidence. Categorical variables were compared using the χ2 or Fisher's exact test, as appropriate. To examine the significance of changes in laboratory markers over time within each group, Wilcoxon signed rank tests were performed. Variables that showed significant differences between the groups on univariate analysis were tested again by multivariate logistic regression analysis. A p value <0.05 was considered to indicate statistical significance.
RESULTS
The study included 795 patients: 178 with iKD, 504 with cKD, and 113 febrile controls. To enable comparison under the same conditions, only KD patients treated with IVIG were included. The most common diagnosis in the febrile control group was viral exanthem, which was observed in 70 patients, followed by pharyngoconjunctival fever (n=25), pharyngitis (n=15), and infectious mononucleosis (n=3). The demographic and clinical characteristics of the subjects are shown in Table 1. Patients in the iKD group were younger than those in the cKD group. Patients in the 2 KD groups experienced more days of fever and more KD-compatible symptoms than the febrile controls. Duration of fever prior to IVIG treatment was longer in the iKD group than in the cKD group, suggesting delayed treatment in the iKD group, but there was no difference in IVIG resistance between the 2. CAAs were more common in the iKD group than in the cKD group.
Table 1. Demographic and clinical characteristics of patients with complete or incomplete KD and of febrile controls.
| Variables | Complete KD (A; n=504) | Incomplete KD (B; n=178) | Febrile controls (C; n=113) | p value | ||
|---|---|---|---|---|---|---|
| A vs. B | A vs. C | B vs. C | ||||
| Male (%) | 292 (57.9) | 107 (60.1) | 70 (61.9) | 0.598 | 0.382 | 0.713 |
| Age (months) | 30.0 (14.0, 46.0) | 26.0 (10.0, 44.0) | 27.5 (15.8, 58.3) | 0.033 | 0.197 | 0.009 |
| Pre-IVIG fever (days) | 5 (4, 5) | 5 (5, 7) | - | <0.001 | - | - |
| Post-IVIG fever (days) | 2 (1, 3) | 2 (1, 4) | - | 0.086 | - | - |
| Total fever (days) | 7 (6, 8) | 7 (6, 9) | 5 (5, 6) | <0.001 | <0.001 | <0.001 |
| Number of symptoms (except fever) | 4 (4, 5) | 3 (2, 3) | 2 (1, 2) | <0.001 | < 0.001 | < 0.001 |
| IVIG resistance (%) | 173 (34.3) | 74 (41) | - | 0.074 | - | - |
| CAA (+) (%) | 40 (7.9) | 29 (16.3) | - | 0.001 | - | - |
Data are expressed as the median Q2 value (25th percentile Q1, 75th percentile Q3).
CAA = coronary artery abnormality; IVIG = intravenous immunoglobulin; KD = Kawasaki disease.
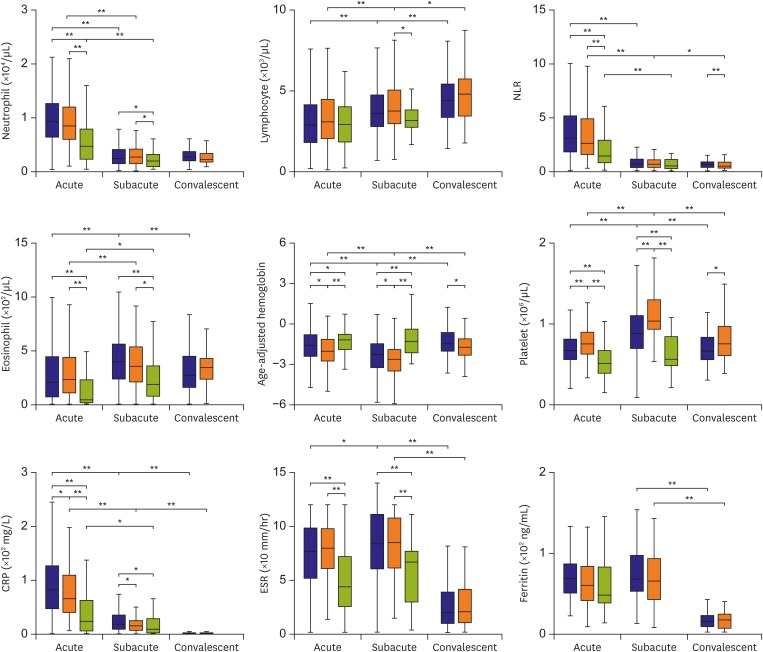
Laboratory markers measured during each phase of disease are summarized in Figure 1. At each phase, neutrophil and eosinophil counts and ESR did not differ significantly between the 2 KD groups, although in both cases all parameters were significantly higher than those in febrile controls. Also, at each phase, the age-adjusted hemoglobin levels were significantly lower and platelet counts and CRP levels significantly higher in the 2 KD groups than in febrile controls. However, comparison of these parameters between the 2 KD groups revealed lower age-adjusted hemoglobin and higher platelet counts in the iKD group, and higher CRP levels in the cKD group. Serum ferritin concentrations at each phase did not differ significantly among the 3 groups.
Figure 1.
Laboratory profiles of IVIG -treated patients with cKD, iKD, and febrile controls. The 3 phases are defined as acute (febrile phase before IVIG), subacute (2 days after defervescence), and convalescent (4–6 weeks after defervescence). Box plots show the median values, with the 25 and 75 percentiles. Ferritin data were obtained from 121 patients with cKD, 51 with iKD, and 22 febrile controls during the acute phase, from 99 patients with cKD and 41 with iKD during the subacute phase, and from 71 patients with cKD and 33 with iKD during the convalescent phase.
cKD = complete Kawasaki disease; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; iKD = incomplete Kawasaki disease; IVIG = intravenous immunoglobulin.
*Indicates a p<0.05, †Indicates a p<0.001.
Over the course of disease, laboratory markers in the 2 KD groups showed similar patterns in Figure 1. Neutrophil counts and NLRs were highest during the acute febrile phase but then decreased, whereas lymphocyte counts were lowest during the acute febrile phase and increased thereafter. Eosinophil counts were lowest during the acute phase and highest during the subacute phase. Low age-adjusted hemoglobin levels during the acute phase became even lower during the subacute phase before increasing again. Platelet counts increased from the acute to the subacute phase and then decreased. CRP levels were highest during the acute phase. A high ESR during the acute phase became even higher during the subacute phase. Most of the laboratory markers of the febrile controls showed patterns similar to those of the 2 KD groups, apart from age-adjusted hemoglobin levels and platelet counts, the levels of which remained steady during the subacute phase.
The clinical and laboratory findings that differed between the KD groups were further analyzed with respect to coronary artery outcome in Table 2. Coronary artery dilation was more common in the iKD group than in the cKD group (13.5% vs. 6.0%, p<0.05), whereas IVIG resistance was associated with coronary dilation only in the cKD group (63.3% vs. 31.9%, p<0.001). For the iKD group, the duration of fever prior to IVIG therapy was longer in patients with a CAA than in those without a CAA (7 vs. 5 days, p=0.005). In the cKD group, the acute NLR was significantly higher in patients with a coronary aneurysm than in those without a CAA (8.7 vs. 3.1, p=0.009). In both KD groups, acute and subacute age-adjusted hemoglobin concentrations were lower and subacute platelet counts higher in patients with coronary artery dilation than in those without a CAA. To identify factors predicting CAA over the course of disease, multivariate analysis was performed by entering significant variables from the univariate analyses in Table 3. IVIG resistance correlated with coronary dilation only in the cKD group (odds ratio [OR], 3.039; p=0.046). Although treatment in the iKD group was delayed more in patients with a CAA than in those without a CAA, total fever days rather than pre-IVIG fever days correlated with the risk of coronary dilation (OR, 1.302; p=0.029). Among laboratory markers, subacute platelet counts in the cKD group (OR, 1.004; p=0.014) and acute and subacute age-adjusted hemoglobin concentrations in the iKD group (OR, 0.538 and 0.583; p=0.006 and 0.018, respectively) predicted coronary dilation. The risk of coronary aneurysm correlated with days of total fever (OR, 1.263; p=0.007) and an acute NLR (OR, 1.059; p=0.044) only in the cKD group.
Table 2. Laboratory markers of patients with complete and incomplete KD according to coronary artery outcome.
| Variable | Complete KD (n=504) | Incomplete KD (n=178) | ||||
|---|---|---|---|---|---|---|
| CAA (−) | Dilation | Aneurysm | CAA (−) | Dilation | Aneurysm | |
| Number (%) | 464 (92.1) | 30 (6)* | 10 (2) | 149 (83.7) | 24 (13.5)* | 5 (2.8) |
| IVIG resistance (%) | 148 (31.9) | 19 (63.3)† | 6 (60) | 60 (40.3) | 11 (45.8) | 3 (60) |
| Pre-IVIG fever (days) | 5 (4, 5) | 5 (4, 5) | 4 (2.75, 7) | 5 (4, 6) | 6.5 (5, 7.5)‡ | 10 (5, 12.5)‡ |
| Total fever (days) | 7 (6, 8) | 7 (5.75, 11.25)‡ | 6.5 (5.75, 14) | 7 (5, 8) | 7 (7, 10.5)‡ | 11 (8.5, 13)‡ |
| Acute NLR | 3.1 (1.8, 5.2) | 2.6 (1.7, 3.9) | 8.7 (2.6, 13.3)‡ | 2.7 (1.6, 4.8) | 2.5 (1.0, 6.4) | 2.6 (1.7, 5.4) |
| Subacute NLR | 0.7 (0.4, 1.1) | 0.9 (0.4, 1.7) | 1 (0.6, 2) | 0.7 (0.4, 1.1) | 0.7 (0.3, 1.4) | 1.1 (0.5, 3.1) |
| Acute age-adjusted hemoglobin | −1.3 (−2.1, −0.4) | −2.0 (−3.2, −1.0)‡ | −1.3 (−1.8, −0.4) | −1.5 (−2.5, −0.9) | −2.1 (−3.2, −1.4)‡ | −2.1 (−4.0, −1.2) |
| Subacute age-adjusted hemoglobin | −2.1 (−2.9, −1.3) | −2.7 (−4.1, −1.7)‡ | −1.9 (−2.4, −1.4) | −2.4 (−3.2, −1.6) | −3.6 (−4.3, −2.5)‡ | −2.3 (−3.3, −2.0) |
| Acute platelet (K/mL) | 331 (275, 396) | 372 (301, 438) | 306 (254, 430) | 373 (314, 443) | 355 (298, 455) | 414 (287, 491) |
| Subacute platelet (K/mL) | 434 (339, 534) | 592 (439, 777)† | 488 (366, 614) | 508 (458, 632) | 612 (500, 827)‡ | 482 (476, 579) |
| Acute CRP (mg/L) | 81 (46, 126) | 99 (59, 145) | 75 (57, 129) | 67 (41, 110) | 44 (33, 96) | 104 (55, 160) |
Data are expressed as number (percentage) or as the median Q2 value (25th percentile Q1, 75th percentile Q3).
CAA = coronary artery abnormality; CRP = C-reactive protein; IVIG = intravenous immunoglobulin; KD = Kawasaki disease; NLR = neutrophil-to-lymphocyte ratio.
*Significantly different between groups with complete and incomplete KD at p<0.05; †Significantly different from CAA (−) group at p<0.001; ‡Significantly different from CAA (−) group at p<0.05.
Table 3. Univariate and multivariate analyses of risk factors according to coronary artery outcome in KD patients.
| Variables | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |||
| Coronary dilation | ||||||||
| Complete KD | IVIG resistance | 3.455 | 1.689–7.068 | 0.001 | 3.039 | 1.154–9.424 | 0.046 | |
| Days of total fever | 1.245 | 1.097–1.391 | <0.001 | 1.037 | 0.622–1.160 | 0.806 | ||
| Acute age-adjusted hemoglobin | 0.653 | 0.502–0.843 | 0.001 | 0.888 | 0.491–1.107 | 0.485 | ||
| Subacute age-adjusted hemoglobin | 0.739 | 0.560–0.975 | 0.032 | 0.989 | 0.641–1.526 | 0.961 | ||
| Acute platelets | 1.004 | 1.001–1.007 | 0.016 | 1.003 | 0.992–1.005 | 0.353 | ||
| Subacute platelets | 1.005 | 1.002–1.006 | <0.001 | 1.004 | 1.001–1.007 | 0.014 | ||
| Incomplete KD | Days of pre-IVIG fever | 1.243 | 1.024–1.517 | 0.03 | 1.016 | 0.944–2.236 | 0.635 | |
| Days of total fever | 1.240 | 1.068–1.443 | 0.007 | 1.302 | 1.093–2.156 | 0.029 | ||
| Acute age-adjusted hemoglobin | 0.649 | 0.455–0.919 | 0.016 | 0.538 | 0.377–0.823 | 0.006 | ||
| Subacute age-adjusted hemoglobin | 0.580 | 0.436–0.907 | 0.01 | 0.583 | 0.420–0.758 | 0.018 | ||
| Subacute platelets | 1.004 | 1.001–1.006 | 0.012 | 1.005 | 0.998–1.012 | 0.14 | ||
| Coronary aneurysm | ||||||||
| Complete KD | Days of total fever | 1.308 | 1.108–1.525 | 0.001 | 1.263 | 1.064–1.484 | 0.007 | |
| Acute NLR | 1.069 | 1.019–1.124 | 0.007 | 1.059 | 1.003–1.120 | 0.044 | ||
| Incomplete KD | Days of pre-IVIG fever | 1.557 | 1.148–2.116 | 0.005 | 1.575 | 0.413–2.423 | 0.244 | |
| Days of total fever | 1.828 | 1.104–1.928 | 0.008 | 1.308 | 0.120–2.852 | 0.606 | ||
| Subacute NLR | 4.946 | 1.088–5.249 | 0.029 | 5.817 | 0.831–40.707 | 0.076 | ||
CI = confidence interval; IVIG = intravenous immunoglobulin; KD = Kawasaki disease; NLR = neutrophil-to-lymphocyte ratio; OR = odds ratio.
DISCUSSION
In this study, although clinical manifestations are incomplete in iKD patients, their laboratory marker profiles in terms of complete blood cell counts and acute phase reactant levels during illness are not intermediate between those of cKD patients and those of febrile controls. This cannot be accounted for by the treatment modalities. The complete blood cell profiles of IVIG-untreated KD patients within the first 20 days of illness are similar to those of IVIG-treated patients.8) IVIG-induced hemolytic anemia can occur after IVIG therapy12),13); however, it is rare and was not observed in the present study. Steroids used to treat IVIG-resistant KD patients can also induce hematological changes; however, because similar proportions of cKD and iKD patients received steroids (3.6% vs. 3.4%), they are unlikely to have had an effect on the conclusions of this study.
Inflammation suppresses erythropoiesis, thereby inducing normocytic and normochromic anemia.14) Because the KD patients in the present study had normocytic and normochromic red blood cells (as did the febrile controls) (data not shown), anemia shown by low age-adjusted hemoglobin levels may indicate the presence of active inflammation. Also, platelet counts are known to increase in proportion to the number of fever days, suggesting that increased counts may reflect a longer period of inflammation.15) During the transition from the acute febrile to subacute afebrile phase, progressions in the patterns of age-adjusted hemoglobin levels and platelet counts of both iKD and cKD patients significantly differ from those of febrile controls. They seem to reflect the presence of ongoing inflammation even after defervescence, which might be KD-specific. However, whether they indicate the presence of inflammation requiring intervention, or slow recovery, is unclear. Considering that levels of age-adjusted hemoglobin and platelet counts mirror the presence of inflammation and the fact that extensive inflammation is a known risk factor for CAA, it is not surprising that KD patients with coronary artery dilation had lower age-adjusted hemoglobin levels and higher platelet counts than KD patients with no CAA. Lower acute age-adjusted hemoglobin levels in iKD patients with coronary dilation could be accounted for the delay in diagnosis, but while cKD patients were timely diagnosed, acute age-adjusted hemoglobin levels in patients with coronary dilation were not different according to the phenotype. Furthermore, multivariate analysis in iKD patients showed that total fever days rather than pre-IVIG fever days correlated with the risk of coronary dilation. In this study, the factors predicting coronary dilation differ according to the phenotype, i.e., lower acute and subacute age-adjusted hemoglobin levels in iKD patients whereas higher subacute platelet counts in cKD patients. The underlying mechanism behind the differences is not clear, but possibly, the nature of the factors that drive inflammation during illness might differ timewise between iKD and cKD patients. Nevertheless, subacute levels of age-adjusted hemoglobin and platelet counts can be used not only as KD-specific markers but also as predictors of coronary dilation in iKD and cKD patients, respectively.
The NLR reflects the balance between inflammation and immune regulation in KD.16) The high acute ratio in cKD patients with a coronary aneurysm suggests a role for neutrophilic inflammation in aneurysm formation. But it was observed only in cKD patients. After fever subsides, the NLRs in this study were about 1 or less regardless of the presence of a CAA and did not differ between cKD and iKD patients. We previously observed that IVIG-resistant febrile KD patients had a high NLR.16) Together, these results suggest that neutrophils are major players during the febrile phase of KD, and that, once fever subsides, the NLR does not predict the risk of a CAA.
A previous study showed that eosinophils preferentially accumulate in epicardial microvessel lesions in patients who die from KD.17) Both the present study and that of Tremoulet et al.8) revealed increases in eosinophil numbers during the acute phase and further increases thereafter. Since patients not treated with IVIG also showed increases in eosinophil numbers after the acute phase (data not shown), this increase does not seem to be related to IVIG treatment. Because we found no difference in eosinophil counts between cKD and iKD patients, or between patients with and without a CAA, the role of eosinophils in KD patients remains unclear.
Although treatment was delayed in iKD patients with CAA, only more days of total fever are associated with the development of CAA in both iKD and cKD patients. In cKD patients specifically, IVIG resistance is a risk factor for CAA not only alone but also because it is associated with a longer duration of total fever, emphasizing the importance of initial intensive treatment if laboratory profiles are predictive of IVIG resistance.18),19),20),21),22)
This study has some limitations. Mainly, this is a retrospective study and is thus subject to bias. We carefully examined all cases and included those by consensus. Also, we only examined blood cell counts and prototypic acute phase reactant levels because other laboratory markers were unavailable for pairwise comparison over time. Nevertheless, laboratory markers we examined in this study are the most easily available and inexpensive markers. Most of laboratory markers were significant only in patients with coronary artery dilation. It is unclear whether these findings are restricted to patients with coronary artery dilation because the small number of patients with a coronary aneurysm limited the power of this study. The roles of neutrophilic inflammation in aneurysm formation in iKD patients need to be defined. Finally, other factors affecting the level of age-adjusted hemoglobin, such as iron reserves, in different ethnic groups should be considered before the age-adjusted hemoglobin is used as a KD-specific marker.
In conclusion, laboratory marker profiles of iKD are not incomplete compared with those of cKD in terms of complete blood cells and acute phase reactants, but the factors predicting coronary dilation differ according to the phenotype, i.e., lower acute and subacute age-adjusted hemoglobin levels in iKD and higher subacute platelet counts in cKD. Further studies are needed to elucidate whether they reflect an intrinsic difference between iKD and cKD patients.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Lee J.
- Investigation: Ha KS, Jang GY, Lee KC, Son CS.
- Methodology: Ha KS.
- Supervision: Lee J.
References
- 1.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 2.Yellen ES, Gauvreau K, Takahashi M, et al. Performance of 2004 American Heart Association recommendations for treatment of Kawasaki disease. Pediatrics. 2010;125:e234–e241. doi: 10.1542/peds.2009-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manlhiot C, Christie E, McCrindle BW, Rosenberg H, Chahal N, Yeung RS. Complete and incomplete Kawasaki disease: two sides of the same coin. Eur J Pediatr. 2012;171:657–662. doi: 10.1007/s00431-011-1631-2. [DOI] [PubMed] [Google Scholar]
- 4.Song D, Yeo Y, Ha K, et al. Risk factors for Kawasaki disease-associated coronary abnormalities differ depending on age. Eur J Pediatr. 2009;168:1315–1321. doi: 10.1007/s00431-009-0925-0. [DOI] [PubMed] [Google Scholar]
- 5.Sudo D, Monobe Y, Yashiro M, et al. Coronary artery lesions of incomplete Kawasaki disease: a nationwide survey in Japan. Eur J Pediatr. 2012;171:651–656. doi: 10.1007/s00431-011-1630-3. [DOI] [PubMed] [Google Scholar]
- 6.Witt MT, Minich LL, Bohnsack JF, Young PC. Kawasaki disease: more patients are being diagnosed who do not meet American Heart Association criteria. Pediatrics. 1999;104:e10. doi: 10.1542/peds.104.1.e10. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Li Q, Zhao XD, et al. Clinical analysis of 942 cases of Kawasaki disease. Zhonghua Er Ke Za Zhi. 2006;44:324–328. [PubMed] [Google Scholar]
- 8.Tremoulet AH, Jain S, Chandrasekar D, Sun X, Sato Y, Burns JC. Evolution of laboratory values in patients with Kawasaki disease. Pediatr Infect Dis J. 2011;30:1022–1026. doi: 10.1097/INF.0b013e31822d4f56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967;16:178–222. [PubMed] [Google Scholar]
- 10.Cha Y. The Korean Society of Hematology, editors. Hematology. 2nd ed. Seoul: Panmun Education; 2011. Classification and diagnosis of red blood cell diseases; p. 49. [Google Scholar]
- 11.Japan Kawasaki Disease Research Committee. Report of subcommittee on standardization of diagnostic criteria and reporting of coronary artery lesions in Kawasaki disease. Tokyo: Ministry of Health and Welfare; 1984. [Google Scholar]
- 12.Comenzo RL, Malachowski ME, Meissner HC, Fulton DR, Berkman EM. Immune hemolysis, disseminated intravascular coagulation, and serum sickness after large doses of immune globulin given intravenously for Kawasaki disease. J Pediatr. 1992;120:926–928. doi: 10.1016/s0022-3476(05)81964-x. [DOI] [PubMed] [Google Scholar]
- 13.Kahwaji J, Barker E, Pepkowitz S, et al. Acute hemolysis after high-dose intravenous immunoglobulin therapy in highly HLA sensitized patients. Clin J Am Soc Nephrol. 2009;4:1993–1997. doi: 10.2215/CJN.04540709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemeth E, Ganz T. Anemia of inflammation. Hematol Oncol Clin North Am. 2014;28:671–681. doi: 10.1016/j.hoc.2014.04.005. [vi.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabharwal T, Manlhiot C, Benseler SM, et al. Comparison of factors associated with coronary artery dilation only versus coronary artery aneurysms in patients with Kawasaki disease. Am J Cardiol. 2009;104:1743–1747. doi: 10.1016/j.amjcard.2009.07.062. [DOI] [PubMed] [Google Scholar]
- 16.Ha KS, Lee J, Jang GY, et al. Value of neutrophil-lymphocyte ratio in predicting outcomes in Kawasaki disease. Am J Cardiol. 2015;116:301–306. doi: 10.1016/j.amjcard.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Terai M, Yasukawa K, Honda T, et al. Peripheral blood eosinophilia and eosinophil accumulation in coronary microvessels in acute Kawasaki disease. Pediatr Infect Dis J. 2002;21:777–781. doi: 10.1097/00006454-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Ashouri N, Takahashi M, Dorey F, Mason W. Risk factors for nonresponse to therapy in Kawasaki disease. J Pediatr. 2008;153:365–368. doi: 10.1016/j.jpeds.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Egami K, Muta H, Ishii M, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. 2006;149:237–240. doi: 10.1016/j.jpeds.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi T, Inoue Y, Takeuchi K, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113:2606–2612. doi: 10.1161/CIRCULATIONAHA.105.592865. [DOI] [PubMed] [Google Scholar]
- 21.Sano T, Kurotobi S, Matsuzaki K, et al. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr. 2007;166:131–137. doi: 10.1007/s00431-006-0223-z. [DOI] [PubMed] [Google Scholar]
- 22.Tremoulet AH, Best BM, Song S, et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. 2008;153:117–121. doi: 10.1016/j.jpeds.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]