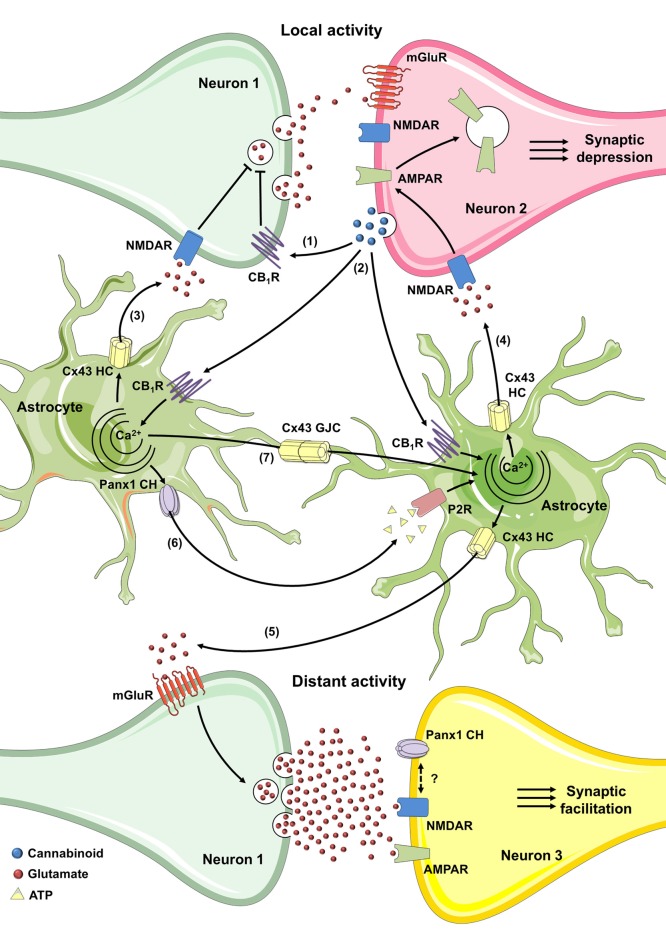
Figure 3.
Possible roles of connexin- and pannexin-based channels in CB-mediated synaptic plasticity through activation of astrocytes. In the hippocampus, the activity-dependent production of eCB triggers a decrease of neurotransmitter release through the stimulation of presynaptic CB1Rs in homoneuronal synapses (1). In the cortex and hippocampus, the coincidence of postsynaptic metabotropic glutamate receptor (mGluR) activation during synaptic activity and Ca2+ influx (not depicted) caused by postsynaptic back-propagating action potentials evoke the release of eCBs (2). The latter stimulates astroglial CB1 receptors and could elicit the Ca2+-dependent release of glutamate or D-serine through Cx43 hemichannels (3), leading to the activation of presynaptic NMDA receptors (NMDARs) and subsequent induction of spike timing dependent long-term depression (t-LTD; Min and Nevian, 2012; Andrade-Talavera et al., 2016). Alternatively, in the hippocampus, astroglial CB1 receptor activation and rise of cytoplasmic Ca2+ may cause the Cx43 hemichannel-dependent release of glutamate (4) which, through the stimulation of postsynaptic NMDAR, elicits the internalization of AMPARs and further t-LTD (Han et al., 2012). At the other end, the eCB-mediated increase in cytoplasmic Ca2+ may trigger the release of glutamate through Cx43 hemichannels (5) that, acting on mGluRs induces lateral potentiation of synaptic transmission at distant synapses (Navarrete and Araque, 2010; Gómez-Gonzalo et al., 2015). The interacting coupling between NMDARs and Pannexin 1 (Panx1) channels could be a possible mechanism to potentiate the above response. Finally, purinergic signaling mediated by ATP released via Panx1 channels (6) along with gap junctional communication (7) among astrocytes could favor the widespread of eCB-mediated intracellular Ca2+ responses.