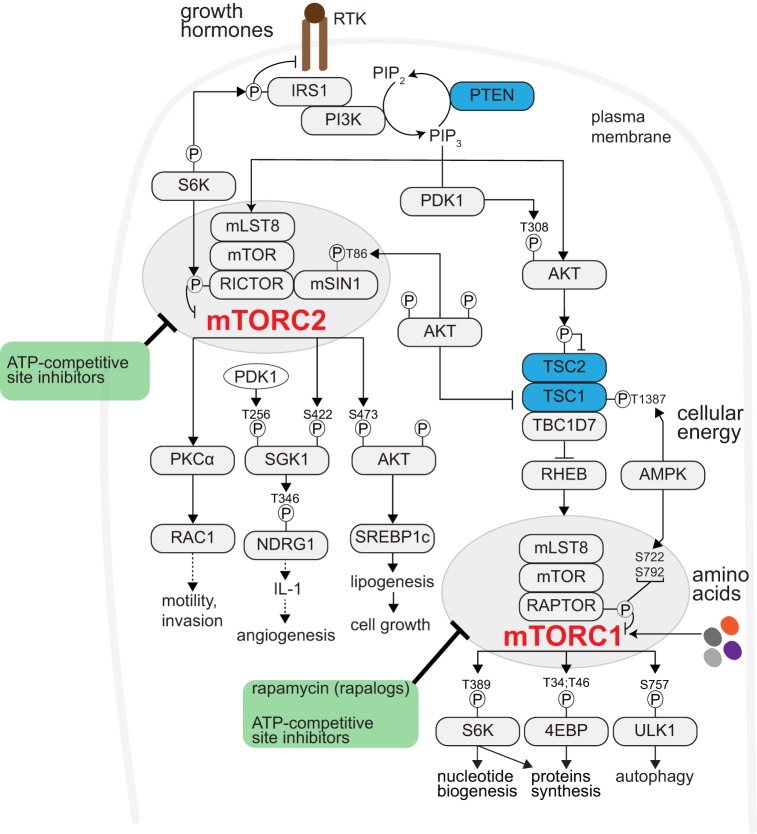
Figure 1.
mTOR signaling promotes anabolism. Receptor Tyrosine Kinases (RTKs)- Phosphatidyl-Inositol-4,5-bisphosphate 3-Kinase (PI3K) activated by growth factor (like insulin). PI3K generates phosphatidylinositol-3,4,5-trisphosphate (PIP3) from the membrane phospholipid phosphatidylinositol-4,5-bisphosphate (PIP2). Phosphatase and Tensin Homolog Deleted on Chromosome 10 (PTEN) counteracts PI3K activity (restoring PIP3 to PIP2). PIP3 recruits to the plasma membrane and activates phosphoinositide-dependent kinase 1 (PDK1) and AKT. PDK1 phosphorylates and activates AKT (pAKT-Thr308). pAKT-Thr308 phosphorylates and inhibits the TSC complex. The TSC complex, composed of tuberous sclerosis complex 1 (TSC1) and TSC2 and TRE2-BUB2-CDC16 domain family member 7 (TBC1D7), activates the lysosomal RAS homolog enriched in brain (RHEB). RHEB interacts with and activates mTORC1. mTORC1 comprises mTOR, mammalian lethal with sec-13 protein 8 (mLST8), and regulatory-associated protein of mammalian target of rapamycin (RAPTOR). mTORC1 can also be activated by nutrients (such as amino acids). Cellular energy status also regulates mTORC1 through AMPK-mediated TSC or RAPTOR phosphorylation. mTORC1 promotes anabolism, among others, through ribosomal protein S6 kinase (S6K), eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4EBP1), and blocks cellular catabolism through Unc-51-like kinase 1 (ULK1). Through S6K-mediated IRS1 phosphorylation, mTORC1 negatively regulates mTORC2-AKT signaling. Rapamycin and its analogs (so-called rapalogues) acutely inhibit mTORC1 allosterically. The ATP-site competitive inhibitor(s) potently block both mTORC1 and mTORC2 signaling. mTORC2 is also activated by RTKs, and consists of mTOR, mLST8, mammalian stress-activated map kinase-interacting protein 1 (mSIN1), and rapamycin-insensitive companion of mTOR (RICTOR). mTORC2 regulates the AGC kinase family members AKT, serum/glucocorticoid-regulated kinase (SGK), and protein kinase C (PKC). Prolonged rapamycin administration may block mTORC2 activity.