Fig. 3.
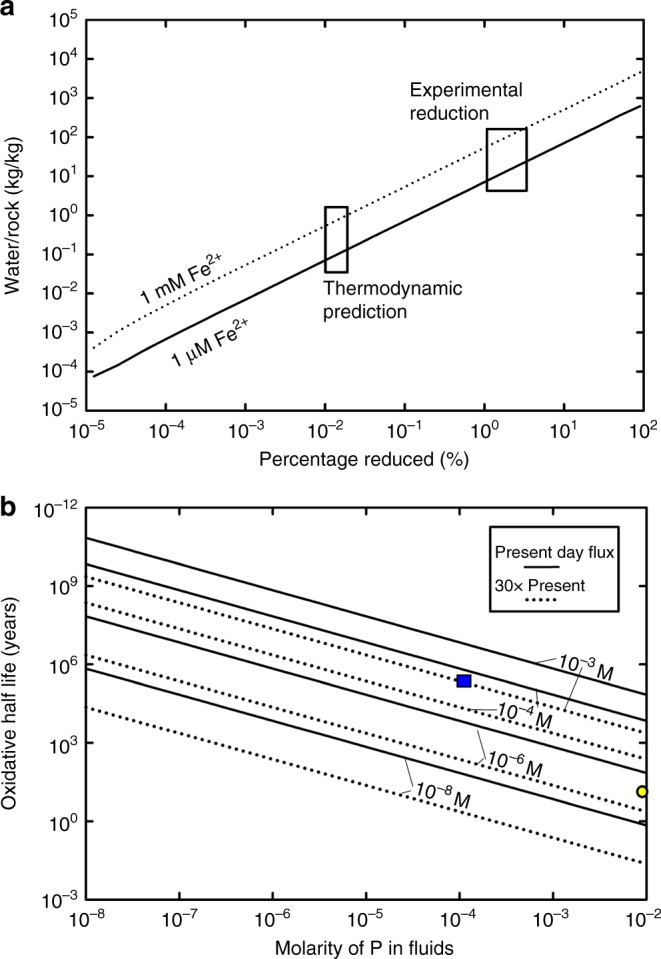
Model results of the extraction of phosphite and its predicted steady-state abundance in an anoxic ocean. a Water-rock ratios required to completely extract phosphite from ferruginous sediments (0.3% P, Fe2+/Fe3+ of 1/1). These calculations assume a total P content of 0.3% by weight, and that the P is in vivianite [Fe3(PO4)2·8H2O] or ferric phosphite—Fe2(HPO3)3. Then, using the solubility data determined experimentally (ED), the quantity of water (pH 7.2) needed to completely dissolve phosphite was determined using the thermodynamic modeling program HSC Chemistry (Methods). Two scenarios were investigated: one where the water in contact with the rock had a starting Fe2+ concentration of 10−6 M, and other, with a concentration of 10−3 M. The amount of phosphite predicted to form from thermodynamics and shown to form from experimental reduction, also imply that lower quantities of phosphite produced at low temperatures (<160 °C) will also be easily extracted at low water-rock ratios. b Steady-state concentration of phosphite predicted within the early oceans, as a function of molarity of phosphite dissolved from rock after diagenesis, oxidative half-life of phosphite for modern day hydrothermal fluid fluxes, and the higher values that might be expected in the Archean28. The blue square corresponds to the predicted phosphite flux from Fig. 3a, with the measured oxidative half-life of Fe(H2PO3)2 under low O2 conditions (Methods). The yellow circle corresponds to the current measurements of half-life oxidation by biology18 with the concentration of phosphite found within the ocean15, which implies a very high flux from sediments or hydrothermal sources, or more likely indicating that oceanic phosphite is not currently steady state, with respect to abiotic sources. This biotic oxidation rate is likely overestimated (Methods)
