Figure 1.
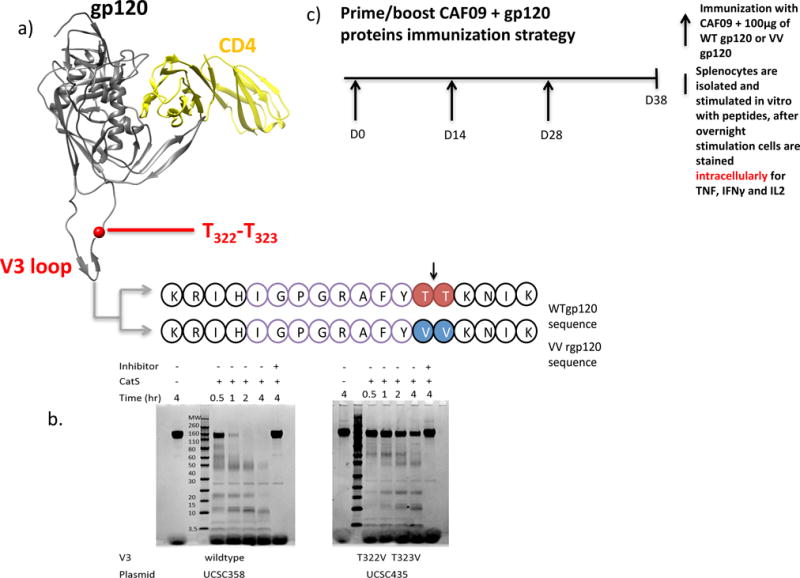
a) Overview of the HIV gp120MN V3 domain. The cathepsin S lysosomal protease cleaves (as indicated by the black arrow) between the amino acids T322T323 (red) in the WT gp120 protein. In the mutant protein that inhibits cathepsin S cleavage, referred to as the VV gp120 protein, the T322T323 has been mutated to V322V323 (blue). The sequence IGPGRAFYTT (purple) is a minimal epitope that has previously been reported to elicit CD8+ T cell responses in BALB/c mice immunized with modified vaccinia virus expressing HIV gp160MN. b) Effect of mutations in the V3 domain of rgp120MN on cathepsin S digestion. Purified rgp120MN and rgp120MN mutated to replace threonine (T) at positions 322 and 323 with valine (V) were digested with cathepsin S (CatS) for the times indicated. The reactions were stopped by the addition of SDS-PAGE sample buffer and stored overnight at −80°C. The samples were loaded onto gels and the protein digests were visualized by Coomassie blue staining. Controls included incubation of the proteins in CatS digest buffer without added enzyme, and incubation in Cat S digest buffer containing the irreversible cathepsin inhibitor Z-FA-FMK. Because there are 4 CatS cleavage sites in rgp120MN the T322V and T323 mutations did not completely prevent protease cleavage. However, N-terminal sequence analysis demonstrated that the fragments detected resulted from cleavages between positions 208 and 209, 261 and 262, and 435 and 436 as described by Yu et al (5). c) The repeated immunization strategy. At day 0 (D0), BALB/c mice were immunized I.P. with 100μg of either wild type (WT) gp120MN or the cathepsin S mutant, VV gp120MN protein in a cationic liposome, CAF09. At 14 day intervals (or at D14 and D28), mice were re-immunized. On D38, splenocytes were isolated and stimulated in vitro with desired peptides for 12 hours (overnight). After 12 hours, splenocytes were stained for intracellular cytokines: TNF, IFNγ and IL-2.
