Abstract
Mantle cell lymphoma (MCL) accounts for 5% to 10% of all non-Hodgkin lymphomas and has the worst prognosis among all lymphomas. The hallmark of MCL is a t(11;14) translocation that results in overexpression of cyclin D1 by tumor cells of virtually all patients. In this study we examined whether cyclin D1 could be an effective tumor-associated antigen for immunotherapy. We identified cyclin D1 peptides for HLA-A*0201 and generated peptide-specific CD8+ T-cell lines from HLA-A*0201+ blood donors and MCL patients. These cell lines proliferated in response to cyclin D1 peptide–pulsed stimulatory cells. Moreover, the T cells efficiently lysed peptide-pulsed but not unpulsed T2 cells and autologous dendritic cells; cyclin D1+ and HLA-A*0201+ human MCL lines MINO, SP53, Jeko-1, and Granta 519; and more importantly, HLA-A*0201+ primary lymphoma cells from MCL patients. No killing was observed with HLA-A*0201− primary lymphoma cells or HLA-A*0201+ normal blood cells, including B cells. These results indicate that these T cells are potent cytotoxic T cells and recognize cyclin D1 peptides naturally presented by patient lymphoma cells in the context of HLA-A*0201 molecules. Taken together, our work identifies cyclin D1 as a potentially important antigen for immunotherapy of MCL.
Keywords: Mantle cell lymphoma, cyclin D1, cytotoxic T lymphocytes, dendritic cells, immunotherapy
INTRODUCTION
Mantle cell lymphoma (MCL) is a well-defined lymphoid neoplasm characterized by a proliferation of a specific population of mature B lymphocytes (1, 2). MCL accounts for 5% to 10% of all non-Hodgkin lymphomas, and is considered an aggressive malignancy (3). MCL is associated with high relapse rates (4) and has the worst prognosis among lymphomas, with a median survival of approximately 3 years. There have been only a few reported long-term survivors (5, 6). Although MCL has been treated with chemotherapy, radiotherapy, hematopoietic stem cell transplantation, molecular targeted therapy, and combination therapy with the above treatments, no standard treatment approach has been established to date (5)5. Therefore, new strategies are needed to improve the overall survival of patients and decrease treatment-associated morbidity.
Adoptive T-cell therapy with antigen-specific T cells is a promising approach for the treatment of cancers (7). The demonstration of autologous idiotype-specific T cells and evidence of clinical response to allogeneic donor lymphocyte infusions indicate that anti-idiotypic cytotoxic T lymphocytes (CTLs) have been generated against human B-cell idiotypes (8–10). Purified autologous immunoglobulin protein has been used as a vaccine for human patients with lymphoma (11). However, despite the elegant nature of the Id vaccine approach, shortcomings of this strategy include the requirement of producing a custom-made protein for each patient and the limitation of the antitumor response to a weak antigen (12). In addition to optimizing immunotherapy methods, there is an urgent need to search for and utilize novel shared lymphoma antigens to efficiently stimulate anti-lymphoma CTL responses in most treated patients.
MCL is genetically characterized by a t(11;14)(q13;q32) translocation that juxtaposes the proto-oncogene CCND1, which encodes cyclin D1, at chromosome 11q13, to the Ig heavy chain gene at chromosome 14q32. As a consequence of the translocation, cyclin D1 becomes constitutively overexpressed in virtually all patients (13). This genetic alteration is thought to be the primary event in the pathogenesis of the tumor (14), probably by facilitating the deregulation of the cell cycle at the G1-S phase transition (13, 15). However, specific lentiviral shRNA-mediated knockdown of cyclin D1 in MCL showed minimal effects on cell survival, suggesting the possibility that cyclin D1 overexpression may represent an initiating event in MCL lymphomagenesis (16). Therefore, the overexpression of cyclin D1 by tumor cells of all MCL patients provides a very attractive antigenic target for immunotherapy aiming at expanding tumor-specific T cells to control MCL growth and proliferation.
Based on these observations, we hypothesized that cyclin D1 may be a potent and universally-expressed tumor-associated antigen (TAA) for MCL. In the present study, we searched for high-affinity cyclin D1 peptides for HLA-A*0201 molecules, and generated and characterized cyclin D1 peptide-specific CTLs obtained from healthy blood donors and lymphoma patients. Our study clearly shows the potential of cyclin D1 as an important antigenic target for anti-lymphoma immunotherapy.
MATERIALS AND METHODS
Tumor cells and patients
Human mantle cell lymphoma lines (HMCLLs) used in these studies include MINO, Granta 519, SP53, and Jeko-1. These cell lines are positive for the t(11;14)(q13; q32) translocation and for HLA-A*0201. Human myeloma cell line ARP-1, which is cyclin D1+ but HLA-A*0201−, was used as a control. All cell lines were maintained in RPMI-1640 medium (Life Technologies, Grand Island, NY) supplemented with 10% heat-inactivated bovine serum, penicillin (10,000 units/mL; Sigma-Aldrich), streptomycin (10 mg/mL; Sigma-Aldrich), gentamicin (50 mg/mL; Sigma-Aldrich), and L-glutamine (29.2 mg/mL; Life Technologies).
Blood and tumor samples were obtained from patients with MCL after they gave informed consent. Buffy coat blood of healthy donors was obtained from our institutional blood bank. Mononuclear cells were separated by Ficoll-Hypaque density centrifugation, and MCL cells were isolated by using anti-CD20 magnetic microbeads (Miltenyi Biotec, Auburn, CA). Purity of isolated tumor cells (CD19+and CD5+ and light chain restriction) was confirmed to be greater than 95% by flow cytometric analysis. Aliquots of purified lymphoma cells and peripheral blood mononuclear cells (PBMCs) were cryopreserved in liquid nitrogen until use. The study was approved by the Institutional Review Board at The University of Texas M. D. Anderson Cancer Center.
Generation of dendritic cells
Monocyte-derived mature DCs were generated from PBMCs by using standard protocols (17–19). Briefly, PBMCs were allowed to adhere in culture flasks for 2 h, and non-adherent cells were collected and cryopreserved for future use. The adherent cells were cultured in Aim-V medium (Invitrogen Co., Grand Island, NY) supplemented with GM-CSF (10 ng/mL) and IL-4 (10 ng/mL, both from R&D Systems, Minneapolis, MN), with further addition of cytokines every other day. After 5 days of culture, DCs were induced to maturation by addition of TNF-α (10 ng/mL) and IL-1β (10 ng/mL, both from R&D Systems) for 48 h.
Immunophenotyping and intracellular cytokine staining
PE- or FITC-conjugated monoclonal antibodies (mAbs) were added to cell pellets, incubated for 30 min on ice, and washed 3 times before analysis. Intracellular cytokine staining was performed by using the Cytofix/Cytoperm kit (BD PharMingen, San Diego, CA). Samples were analyzed by using a flow cytometer (FACSCaliber; Becton Dickinson, Mountain View, CA).
Immunohistochemistry
For immunohistochemical evaluation, lymph node tissues were stained by using mAb specific for human cyclin D1 (GeneTex, Inc., San Antonio, TX). Slides were counterstained with Harris hematoxylin and examined by standard light microscopy. Samples were analyzed by using an Olympus BX51TF microscope equipped with UPlan FL ×40/0.75 and ×20/0.50 objective lenses (Olympus). Pictures were taken by using Olympus QColor3 and analyzed by using QCapture 2.60 software (QImaging).
Western blot analysis
Western blot analysis was used to detect cyclin D1 protein expression in HMCLLs. Cell lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis. After transfer to nitrocellulose membrane and subsequent blocking, the membranes were immunoblotted with mouse anti-human cyclin D1 antibody (Cell Signaling Technology, Inc., Beverly. MA) and visualized with HRP–conjugated donkey anti-goat IgG antibody (Santa Cruz Biotechnology, Santa Cruz, CA), followed by enhanced chemiluminescence (Pierce Biotechnology, Rockford, IL) and autoradiography.
Peptide-T2 cell binding assay
All peptides were synthesized in the Protein Chemistry Core Laboratory at Baylor College of Medicine. The purity of synthetic peptides was confirmed to be greater than 98% by reverse-phase high performance liquid chromatography and mass spectrometry. Synthetic peptides were dissolved in dimethylsulfoxide (DMSO; Sigma), and stored at 20°C until use.
Peptides binding to HLA-A*0201 molecules were measured by using the T2 cell line according to a protocol previously described (20). Briefly, T2 cells were incubated overnight with 3 µg/mL of β2-microglobulin (Sigma) and different concentrations of peptides, followed by washing and incubation with FITC-labeled anti-HLA-A*0201 mAb BB7.2 (BD PharMingen). After washing, cells were analyzed for the levels of HLA-A*0201 expression by flow cytometry. HLA-A*0201 expression was quantified according to the formula [(mean fluorescence with peptide − mean fluorescence without peptide)/mean fluorescence without peptide] × 100%.
Generation of Cyclin D1-specific CD8+ T-cell lines
Cyclin D1-specific T cells were generated from PBMCs of HLA-A*0201+ blood donors and MCL patients by repeated stimulation of autologous T cells with peptide-loaded mature DCs, as we have reported previously for other TAAs (21, 22). Briefly, the non-adherent cells of PBMCs (2 × 106/mL; used as a T-cell population) were cocultured in 50 mL tissue-culture flasks at 37°C in 5% CO2 for 7 to 10 days in Aim-V medium supplemented with 10% pooled human serum (T-cell medium) with mature DCs (2 × 105/mL) preincubated with cyclin D1 peptides at a final concentration of 20 µg/mL at 37°C for 2 h. After culture, T cells were collected and restimulated with cyclin D1 peptide-pulsed autologous mature DCs every week, and the cultures were fed every 5 days with fresh medium containing recombinant IL-2 (20 IU/mL), IL-7 (5 ng/mL) and IL-15 (5 ng/mL; all from R&D System). Induction of cyclin D1-specific T cells was monitored weekly by using a T-cell proliferation assay and cyclin D1 peptide-HLA-A*0201 tetramers (synthesized by the MHC Tetramer Laboratory, Baylor College of Medicine, Houston, TX). After 5 to 6 cycles of in vitro stimulation and selection, T-cell lines were established and expanded in T-cell medium containing IL-2 (100 IU/mL), IL-7 (5 ng/mL), and IL-15 (5 ng/mL) for 2 weeks and subjected to functional tests.
Proliferation assays
T cells (5 × 104/100 µL/well) were seeded on 96-well U-bottom tissue culture plates (Corning Incorporated, Corning, NY) in T-cell medium. Varying numbers of autologous mature DCs loaded with or without cyclin D1 peptides were added to the plates and cultured for 4 days at 37°C in 5% CO2. T-cell proliferation was measured after overnight incubation with 3[H]-thymidine (0.5 µCi/0.037 MBq/well). Results are expressed as mean count per minute (CPM) of triplicate cultures. In some experiments, cultured T cells were labeled with 5(6)-carboxyfluorescein diacetate succinimidyl ester (CFSE, 5 µM; Molecular Probes, Eugene, OR) for 10 minutes at 37°C. After washing, labeled T cells were seeded on 24-well plates and incubated with various numbers of autologous mature DCs loaded with or without cyclin D1 peptides for 5 days. Flow cytometry analysis was used to detect dilutions of CFSE (23).
Cytotoxicity assay
The standard 4-h 51Cr-release assay (22) was performed to measure cytolytic activity of the T cells against target cells, including autologous DCs or T2 cells loaded with or without peptides, HMCLLs, and primary MCL cells isolated from patients. Target cells were incubated with 100 µCi of 51Cr-sodium chromate for 1 h, washed extensively, seeded (1 × 104 cells/well) on 96-well U-bottom plates in T-cell medium, and cocultured for 4 h with various numbers of T cells. All assays were performed in triplicate. Results are expressed as mean percentages of 51Cr release calculated as follows: [(sample counts − spontaneous counts)/(maximum counts − spontaneous counts)] × 100%. Spontaneous release was less than 20% of the maximum 51Cr uptake.
To determine whether the cytolytic activity was restricted by major histocompatibility complex (MHC) class I or II molecules, 20 µg/mL mAbs against HLA-ABC (W6/32) or HLA-A*0201 (BB7.2; both from Serotec Ltd., Oxford, UK), HLA-DR (B8.12.2; Immunotech, Marseilles, France), or isotypic controls (Immunotech) were added to the cultures at the start of the assay.
ELISPOT assay
Detailed methods of the ELISPOT assay for enumeration of antigen-specific, IFN-γ-secreting cells have been previously described (24), (25). The number of IFN-γ spots was enumerated by an automate ImmunoSpot analyzer (Cellular Technology Ltd, Cleveland, OH). All samples were run in duplicate. Data are expressed as the mean number of IFN-γ-secreting cells/104 T cells.
Statistical analysis
The Student t test was used to compare various experimental groups. A P value less than .05 was considered statistically significant. Unless otherwise indicated, means and standard deviation (SD) are shown.
RESULTS
Cyclin D1 is overexpressed in MCL but not in normal B cells
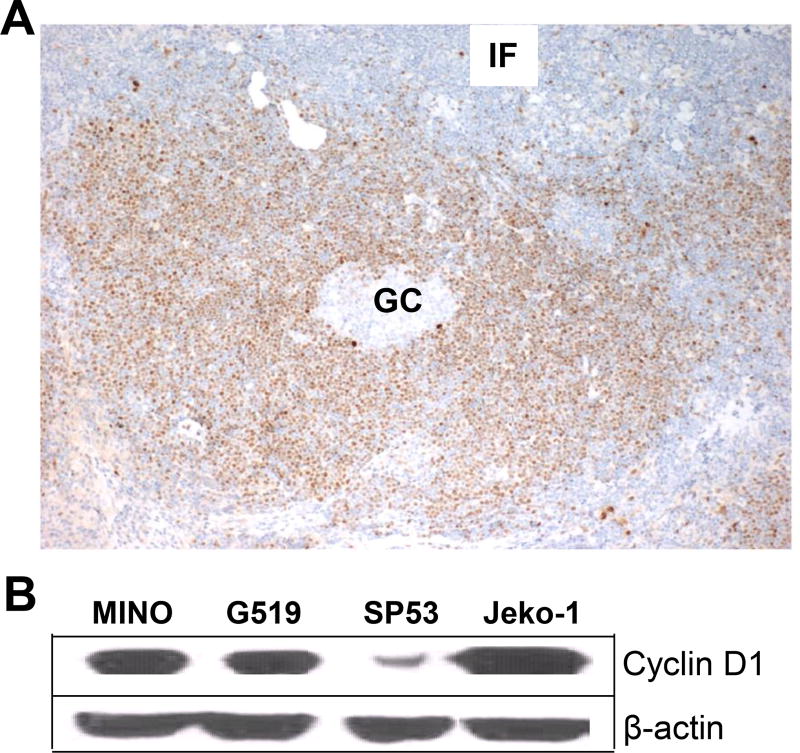
Immunohistochemistry and Western blot analyses were used to examine the expression of cyclin D1 in primary MCL in lymph node samples of patients and in established HMCLLs, respectively. As shown in Figure 1A, cyclin D1 protein was only detected in lymphoma cells with a typical mantle zone pattern but not in normal B cells located in the germinal center and interfollicular area. Cyclin D1 protein was also detected in all 4 HMCLLs (Figure 1B). These results confirm the universal expression of cyclin D1 in MCL cells.
Figure 1.
Expression of cyclin D1 in primary MCL and established cell lines. (A) Immunohistochemistry staining showing the expression of cyclin D1 by lymphoma cells with a predominantly mantle zone pattern, while normal B cells and other lymph node cells in the germinal center (GC) and the interfollicular area (IF) are negative for cyclin D1. The photomicrographs shown are at ×100 magnification. A representative staining of one lymph node sample from a MCL patient out of 4 examined is shown. (B) Western blots showing cyclin D1 protein expression in HMCLLs MINO, SP53, Jeko-1, and Granta 519 (G519). Representative result of one experiment out of 3 performed is shown.
Selection of cyclin D1 peptides
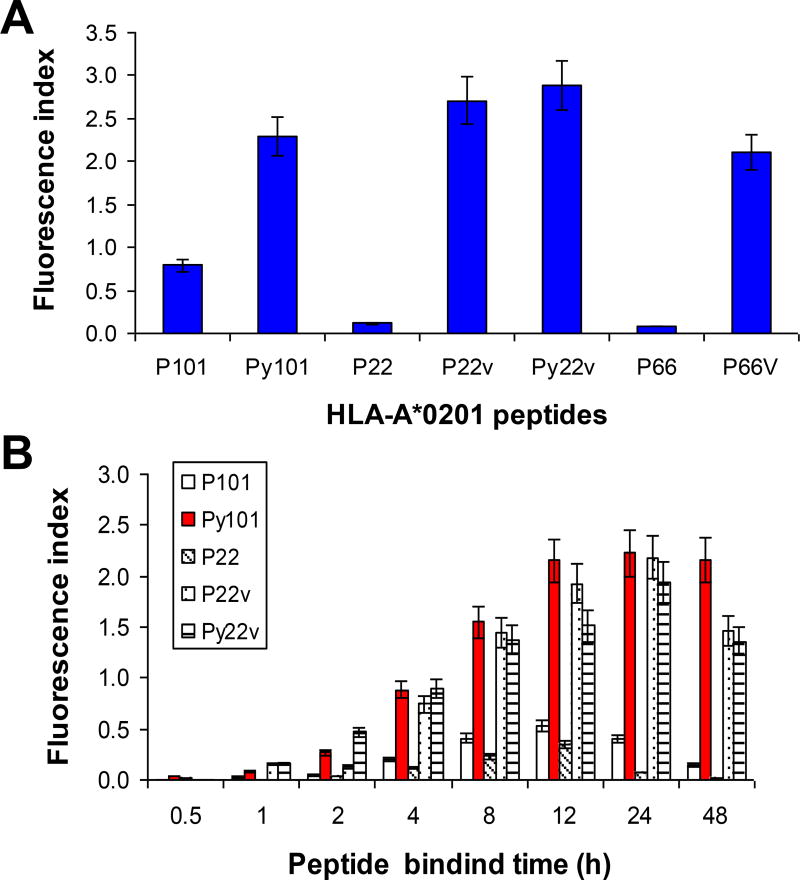
The sequence of human cyclin D1 was reviewed for peptides that could potentially bind to HLA-A*0201 by using a peptide binding database (http://www.bimas.dcrt.nih.gov), and several peptide sequences were obtained. After comparing the predictive binding scores, we identified and selected two peptides that could potentially bind HLA-A*0201 molecules (P101 and P22). Heteroclitic peptides, by replacing position-1 amino acid with tyrosine and/or C-terminal amino acid with valine (26, 27), were made (P101 to Py101, and P22 to P22v and Py22v), which had significantly higher predictive binding scores than the original ones (Table 1). A Peptide-T2 binding assay confirmed that heterolitic peptides have higher binding affinity (Figure 2A) and binding stability (Figure 2B). Dickkopf-1 (DKK1) peptide P66 and its heterolitic peptide P66v (21) were used as controls in binding affinity studies presented in Figure 2A.
Table 1.
Potential cyclin D1 and control peptides for HLA-A*0201 molecules
| Name | Sequence | Position | Predictive binding scoreaa |
Fluorescence Indexbb |
|---|---|---|---|---|
| Cyclin D1-P101 | LLGATCMFV | 101 | 650 | 0.40 |
| Cyclin D1-Py101 | YLGATCMFV | 101 | 1760 | 2.22 |
| Cyclin D1-P22 | LLNDRVLRA | 22 | 79 | 0.07 |
| Cyclin D1-P22v | LLNDRVLRV | 22 | 1115 | 2.19 |
| Cyclin D1-Py22v | YLNDRVLRV | 22 | 3017 | 1.94 |
| DKK1-P66 | ILYPGGNKY | 66 | 0.4 | 0.08 |
| DKK1-P66v | ILYPGGNKV | 66 | 378 | 2.36 |
| Flu matrix | GILGFVFTL | 58 | 550 | 1.55 |
| HIV pol | ILKEPVHGV | 476 | 39 | 1.26 |
Estimate of half-time of disassociation and calculated score in arbitrary units.
(Mean fluorescence with peptide-mean fluorescence without peptide)/(mean fluorescence without peptide). Results are representative of two experiments.
Figure 2.
Binding affinity and stability of cyclin D1 peptides with HLA-A*0201 molecules. Peptide binding assay showing (A) binding affinity and (B) stability (fluorescence index) of two cyclin D1 peptides P101 and P22 and their heteroclitic peptides Py101, P22v, and Py22v for HLA-A*0201 molecules. DKK1 peptide P66 and its heteroclitic peptide P66v were used as controls in (A). In (A) T2 cells were incubated with 100 µg/mL peptides overnight, and in (B) T2 cells were incubated with 100 µg/mL peptides for different time points, and analyzed for surface HLA-A*0201 expression. Details are provided in the Methods section. Representative results of three independent experiments are shown.
Generation of cyclin D1 peptide-specific CD8+ T-cell lines
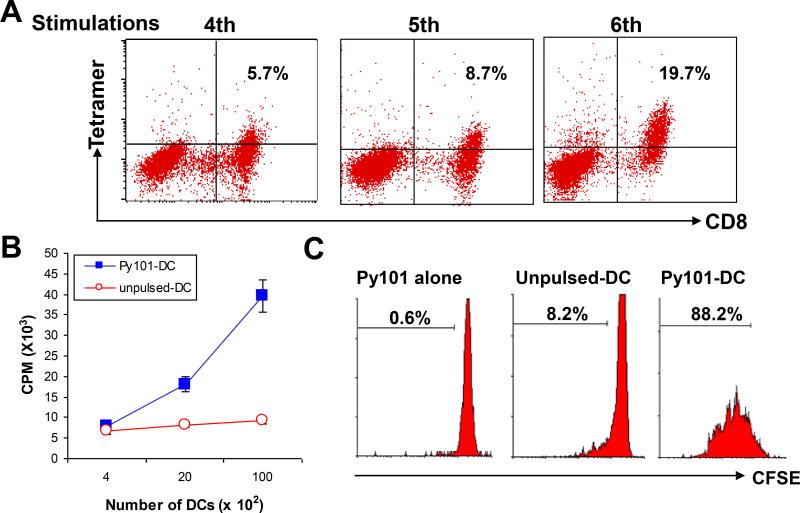
As the solubility of the Py101 peptide was better than that of P22 heterolitic peptides, we chose to use the Py101 cyclin D1 peptide as an immunogen to propagate cyclin D1-specific T cells in this study. To generate cyclin D1 peptide-specific T cells from HLA-A*0201+ blood donors and MCL patients, autologous mature DCs pulsed with the Py101 peptide were used as antigen-presenting cells. After 5 to 7 rounds of in vitro stimulation, T-cell lines were obtained, which contained about 45% CD4+ and 55% CD8+ T cells. As exemplified by the results of peptide-tetramer staining, the frequencies of peptide-specific CD8+ T cells increased during in vitro stimulation; from 5.7% of specific T cells at fourth stimulation to 19.7% at sixth stimulation (Figure 3A). The T-cell lines proliferated in response to autologous DCs pulsed (but not unpulsed) with cyclin D1 peptide Py101 (P < .01, compared with unpulsed DC), as measured by 3[H]-thymidine incorporation (Figure 3B) and CFSE-dilution (Figure 3C) assays. These results indicated that the T cells are indeed specific for the cyclin D1 Py101 peptide.
Figure 3.
Generation of cyclin D1 peptide-specific T-cell lines. (A) Frequency of cyclin D1 Py101 peptide-specific CD8+ T cells measured by HLA-A*0201 peptide-tetramer staining in cultures during in vitro stimulations. Proliferative responses measured by (B) 3[H]-thymidine incorporation or (C) CFSE dilution assays of T cells specific for Py101 peptide in response to autologous Py101 peptide-pulsed (Py101-DC) or unpulsed DCs. Figures inside dot plots or histograms represent the percentages of T cells. Shown are the results of a T-cell line generated from a HLA-A*0201+ blood donor. Similar results are obtained with other Py101 peptide-specific T-cell lines from HLA-A*0201+ blood donors or MCL patients.
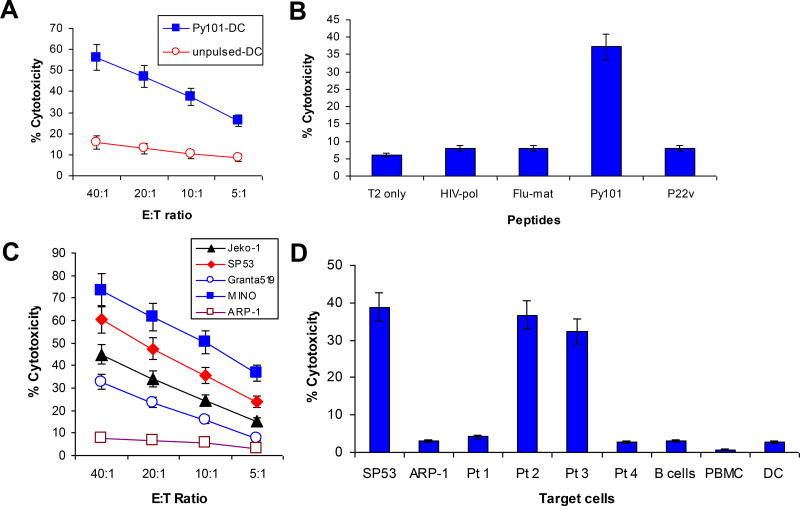
Cytolytic activity of cyclin D1-specific CD8+ T cells
The standard 51Cr-release assay was used to examine the cytotoxicity of the T cells against different target cells. First, our results show that the T cells specifically lysed autologous DCs pulsed, but not unpulsed, with the cyclin D1 peptide Py101 (Figure 4A). Next, we assessed the cytotoxic activity of the T cells against T2 cells pulsed with cyclin D1 and control peptides, and showed that these T cells specifically lysed T2 cells pulsed with cyclin D1 Py101 (P < .01, compared with control peptides) but not unpulsed T2 cells or T2 cells pulsed with irrelevant peptides, which included HIV type 1 reverse transcriptase (HIV-pol) (28), influenza virus matrix protein (Flu-matrix) (29), and cyclin D1 P22v (Figure 4B). These results further confirm the specificity of the T cells.
Figure 4.
Cytolytic activity of cyclin D1 peptide-specific T cells. Shown is the cytotoxicity of cyclin D1 Py101-specific T cells against: (A) Autologous DCs pulsed with or without Py101 peptide; (B) T2 cells pulsed with or without different peptides, including cyclin D1 Py101 and P22v, or control peptides HIV-pol and Flu-matrix (Flu-mat); (C) HMCLLs SP53, MINO, Grant 519, and Jeko-1, and a myeloma cell line ARP-1; and (D) Primary lymphoma cells from four patients with MCL and autologous normal blood cells including DCs, B cells and PBMCs. Patients #2 and #3 were HLA-A*0201+, and patients #1 and #4 were HLA-A*0201−. All primary MCL cells expressed cyclin D1 protein. In D, SP53 and ARK-1 were used as positive and negative controls, respectively. An effector:target (E:T) ratio of 10:1 was used in B and D. Shown are the results of a T-cell line generated from a HLA-A*0201+ MCL patient (#2). Similar results have been obtained with other Py101 peptide-specific T-cell lines from HLA-A*0201+ blood donors or MCL patients.
We proceeded to examine the cytolytic activity of the T cells against lymphoma cells, including HMCLLs and primary lymphoma cells from MCL patients. As shown in Figure 4C, the T cells effectively lysed all 4 HMCLLs (MINO, SP53, Jeko-1, and Granta519 cells) that are HLA-A*0201+ and express cyclin D1. No killing was observed against cyclin D1+/HLA-A*0201− myeloma cell line ARP-1 cells. Furthermore, the T cells efficiently killed cyclin D1+/HLA-A*0201+ primary lymphoma cells from patients #2 and #3 but not lymphoma cells from patients #1 and #4 who are cyclin D1+ but HLA-A*0201− (Figure 4D). We also examined whether the T cells were cytolytic to normal hematopoietic cells. In these experiments, autologous mature DCs, blood B cells (purified by using anti-CD19 antibody-coated magnetic microbeads), and PBMCs were used as target cells. As shown in Figure 4D, the T cells did not kill HLA-A*0201+ DCs, B cells or PBMCs. Together, these results demonstrate that the T cells are not only able to lyse cyclin D1 peptide-pulsed DCs and T2 cells, but also lymphoma cells, which included primary lymphoma cells from HLA-A*0201+ MCL patients. These findings indicate that the T cells are potent and cyclin D1-specific CTLs, and that the cyclin D1 peptide is naturally presented in the context of HLA-A*0201 molecules by lymphoma cells and shared among patients.
MHC restriction and cytolytic pathways of the T cells
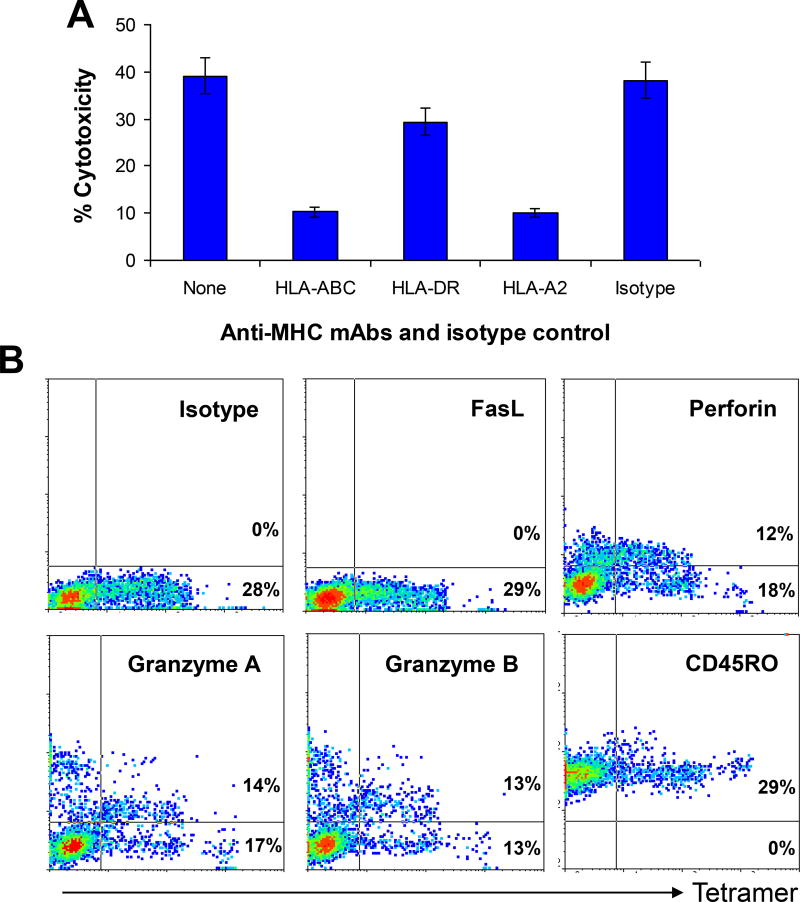
To determine MHC restriction of the T-cell–mediated cytotoxicity, we examined the inhibitory effects of anti-MHC mAbs. As shown in Figure 5A, mAbs against HLA-ABC or HLA-A*0201 significantly (70–80%) inhibited T-cell–mediated cytotoxicity against lymphoma cells (P < .01, compared with medium control). No inhibitory effect was observed with mAb against HLA-DR or isotype control IgG. The results indicate that the cytotoxicity is attributed to MHC class I, and more specifically, HLA-A* 0201-restricted CD8+ T cells.
Figure 5.
MHC restriction and cytolytic pathways of the T cells. (A) Inhibition of T-cell–mediated cytotoxicity against SP53 lymphoma cells by mAbs against MHC class I (HLA-ABC), MHC class II (HLA-DR), or HLA-A*0201 (HLA-A2). Isotypic IgG and cultures with medium without addition of the mAbs or isotypic IgG served as controls. An effector:target (E:T) ratio of 10:1 was used. (B) Flow cytometry analysis showing the expression of FasL, granzyme B, granzyme A, perforin, and CD45RO by HLA-A*0201-Py101 tetramer+ T cells. Shown are the results of gated CD8+ T cells of a T-cell line generated from a HLA-A*0201+ blood donor. Similar results were obtained with other Py101 peptide-specific T-cell lines from HLA-A*0201+ blood donors or MCL patients.
Flow cytometry analysis was used to examine the expression of granzyme B, granzyme A, perforin, and Fas ligand (FasL) by the T cells. These results indicate that the CTLs may kill their target cells via the perforin/granzyme pathways, because they expressed perforin and high levels of granzyme B and granzyme A but not FasL (Figure 5B). The T cells also expressed CD45RO, indicating that they were memory effector cells (30–32).
Expression and production of IFN-γ by the T cells
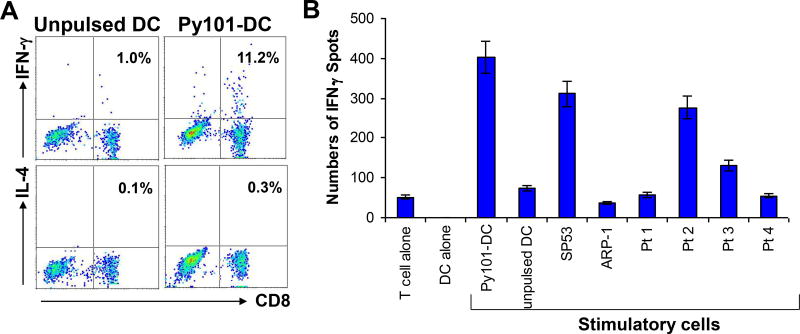
Two independent methods were used to examine the cytokine expression profiles of the T cells. Figure 6A shows a representative experiment of intracellular cytokine staining for IFN-γ and IL-4 expression in Py101-specific T cells. Upon restimulation with DCs pulsed with Py101 peptide, but not with unpulsed DCs, 11.2% of the T cells expressed IFN-γ. IL-4–expressing T cells were very few (0.3%). To detect cytokine secretion, an ELISPOT assay was used to quantitate IFN-γ-secreting cells. After restimulation with DCs pulsed with cyclin D1 peptide, or with cyclin D1+/HLA-A*0201+ SP53 and cyclin D1+/HLA-A*0201+ primary lymphoma cells from patients #2 and #3, large numbers of IFN-γ–secreting cells were detected among the T cells (Figure 6B). Other stimulatory cells, such as unpulsed DCs, primary lymphoma cells from patients #1 and #4 (HLA-A*0201−), and HLA-A*0201− ARP-1 myeloma cells did not increase the number of IFN-γ-secreting cells among the T cells. Taken together, the results showed that Py101-specific T cells express IFN-γ but not IL-4, and thus were identified as type-1 T cells (32, 33).
Figure 6.
Cytokine expression profiles of the T cells. (A) Intracellular cytokine staining showing the percentages of IFN-γ and IL-4-expressing CD8+ T cells in a Py101-specific T-cell line after restimulation with unpulsed DCs or DCs pulsed with Py101 peptide; and (B) ELISPOT assay showing the numbers of IFN-γ-secreting cells per 104 T cells in a Py101-specific T-cell line induced by unpulsed DCs or DCs pulsed with Py101 (DC-Py101), tumor cell lines SP53 and ARP-1, and primary lymphoma cells from 4 MCL patients (Pt 1–Pt 4). Controls include cultures with T cells or DCs alone. Shown are the results of a T-cell line generated from a HLA-A*0201+ MCL patient (#2). Similar results were obtained with other Py101 peptide-specific T-cell lines from HLA-A*0201+ blood donors or MCL patients.
DISCUSSION
Many, if not most, TAAs identified thus far are normal “self” proteins aberrantly expressed by the tumor cells (34, 35), which nevertheless are capable of being presented on human MHC class I and/or II molecules and stimulating host T cells. An ideal TAA is: 1) universally expressed by cancer cells of different origin, 2) undetectable or low in normal tissues, and 3) vital for tumor cell growth and/or survival so that the tumors cannot avoid immune attack simply by not expressing the antigen or by downregulating it. The best examples of such a TAA include the telomerase catalytic subunit (hTERT) (36, 37)34, 35 and survivin (38, 39). Preclinical (36–39)and clinical Phase I studies (40–43)using these TAAs have generated promising results, with minimal toxicity and potential efficacy. Cyclin D1 may belong to this category of TAAs. Due to a chromosomal t(11;14)(q13;q32) translocation, overexpression of cyclin D1 is present in the lymphoma cells of almost 100% of patients with MCL, whereas the expression of cyclin D1 in normal cells is low and transient (14, 44, 45). Our own study has confirmed the expression of cyclin D1 in all MCL samples that we examined. Furthermore, cyclin D1 expression and activity is crucial for MCL growth since it is a well-known cell cycle regulator and promotes G1- to S-phase cell cycle progression (46). Hence, MCL cells cannot cease to express or downregulate the expression of cyclin D1 to escape immune attack.
The goal of the present study was to examine whether cyclin D1 is a suitable TAA for immunotherapy in MCL. To generate cyclin D1-specific CTLs, we identified and synthesized the top two candidate cyclin D1 peptides P101 and P22 after searching cyclin D1 sequence for HLA-A*0201 binding motifs. To enhance their binding affinity, heteroclitic peptides (26, 27)for P101 (Py101) and for P22 (P22v and Py22v) were synthesized. All of the heteroclitic peptides had increased binding affinity compared with the original peptides (Table 1). In this study, we used Py101 as cyclin D1 antigen to generate cyclin D1-specific CTLs. From HLA-A*0201+ healthy donors and MCL patients, several cyclin D1 Py101-specific T-cell lines were obtained. These T cells proliferated in response to and were cytolytic against Py101-pulsed T2 and autologous DCs, but not to the unpulsed cells. Also, these T cells efficiently lysed cyclin D1+/HLA-A*0201+ HMCLLs MINO, SP53, Jeko-1, and Granta 519, and more importantly, primary lymphoma cells from HLA-A*0201+ MCL patients. As expected, these CTLs did not kill normal autologous blood cells, including DCs, B cells, and PBMCs, or primary lymphoma cells from HLA-A*0201− MCL patients. These findings indicate that the T cells are potent CTLs that recognize cyclin D1 peptides naturally processed and presented by lymphoma but not normal blood cells in the context of surface MHC class I (HLA-A*0201) molecules. Our study also suggests that cyclin D1-specific CTLs may be promising effector cells for immunotherapy of MCL.
Because we started with CD3+ T cells in generating cyclin D1 Py101-specific CTLs, our cell lines contained both CD4+ and CD8+ T cells. However, it is evident that CD8+ T cells in the cell lines were cyclin D1-specific T cells because the cytolytic activity of the T cells was blocked by mAbs against HLA-ABC and HLA-A*0201 but not by mAb against MHC class II molecules. By using different immune assays, we characterized the T cells. Phenotypically, the gated CD8+ T cells expressed CD45RO, perforin and granzymes B and A, but not FasL, indicating that they were memory effector CTLs that may mediate their cytolytic activity via the granzyme-perforin pathways. Functionally, the T cells secreted IFN-γ but not IL-4, and displayed strong cytotoxic activity against their target cells, indicating that they are type-1 CTLs (32, 33). CD8+ CTLs constitute one of the most important arms of the immune system, with the capacity to recognize and destroy cancerous cells (47). Thus, these CTLs could be used to eliminate residual MCL cells remaining after initial frontline therapies such as rituximab and/or combination chemotherapy regimens (48, 49).
In conclusion, our study demonstrates that cyclin D1 peptide-specific CTLs can be generated from both healthy blood donors and MCL patients by stimulating autologous T cells with DCs pulsed with cyclin D1 peptides. These CTLs may be promising effector cells for immunotherapy in MCL because they are potent killer cells that are able to specifically and effectively lyse autologous primary lymphoma cells from MCL patients but not normal blood cells in vitro. This study provides preliminary evidence to support our hypothesis that cyclin D1 may be used as universal vaccines to immunize patients with MCL. Thus, our study lays a foundation for additional preclinical and clinical studies to evaluate the safety, feasibility, and efficacy of cyclin D1-based immunotherapy in the treatment of the patients.
Acknowledgments
We thank Alison Woo for providing editorial assistance.
Grant support: This work was supported by institutional start-up funds from The University of Texas M. D. Anderson Cancer Center, National Cancer Institute grants (R01 CA96569 and R01 CA103978), Commonwealth Foundation for Cancer Research, and funds from the Crutchfield family and the Kimmel family philanthropic foundations.
Footnotes
AUTHORSHIP
Contribution: QY conceptualized the research and MW, LS, and QY initiated the work, designed the experiments, and wrote the paper. LS, JQ, XH, ZC, and LZ performed the experiments and statistical analyses, and PL performed immunohistochemistry analyses.
References
- 1.Pinyol M, Bea S, Pla L, Ribrag V, Bosq J, Rosenwald A, et al. Inactivation of RB1 in mantle-cell lymphoma detected by nonsense-mediated mRNA decay pathway inhibition and microarray analysis. Blood. 2007;109:5422–5429. doi: 10.1182/blood-2006-11-057208. [DOI] [PubMed] [Google Scholar]
- 2.Zelenetz AD. Mantle cell lymphoma: an update on management. Ann Oncol. 2006;17(Suppl 4):iv12–14. doi: 10.1093/annonc/mdj992. [DOI] [PubMed] [Google Scholar]
- 3.Martin P, Leonard JP. Novel therapeutic targets in mantle cell lymphoma. Expert Opin Ther Targets. 2007;11:929–940. doi: 10.1517/14728222.11.7.929. [DOI] [PubMed] [Google Scholar]
- 4.Evens AM, Winter JN, Hou N, Nelson BP, Rademaker A, Patton D, et al. A phase II clinical trial of intensive chemotherapy followed by consolidative stem cell transplant: long-term follow-up in newly diagnosed mantle cell lymphoma. Br J Haematol. 2008;140:385–393. doi: 10.1111/j.1365-2141.2007.06908.x. [DOI] [PubMed] [Google Scholar]
- 5.Bertoni F, Ponzoni M. The cellular origin of mantle cell lymphoma. Int J Biochem Cell Biol. 2007;39:1747–1753. doi: 10.1016/j.biocel.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Campo E, Raffeld M, Jaffe ES. Mantle-cell lymphoma. Semin Hematol. 1999;36:115–127. [PubMed] [Google Scholar]
- 7.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osterroth F, Garbe A, Fisch P, Veelken H. Stimulation of cytotoxic T cells against idiotype immunoglobulin of malignant lymphoma with protein-pulsed or idiotype-transduced dendritic cells. Blood. 2000;95:1342–1349. [PubMed] [Google Scholar]
- 9.Schultze JL, Seamon MJ, Michalak S, Gribben JG, Nadler LM. Autologous tumor infiltrating T cells cytotoxic for follicular lymphoma cells can be expanded in vitro. Blood. 1997;89:3806–3816. [PubMed] [Google Scholar]
- 10.Wen YJ, Barlogie B, Yi Q. Idiotype-specific cytotoxic T lymphocytes in multiple myeloma: evidence for their capacity to lyse autologous primary tumor cells. Blood. 2001;97:1750–1755. doi: 10.1182/blood.v97.6.1750. [DOI] [PubMed] [Google Scholar]
- 11.Kwak LW, Campbell MJ, Czerwinski DK, Hart S, Miller RA, Levy R. Induction of immune responses in patients with B-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors. N Engl J Med. 1992;327:1209–1215. doi: 10.1056/NEJM199210223271705. [DOI] [PubMed] [Google Scholar]
- 12.Franki SN, Steward KK, Betting DJ, Kafi K, Yamada RE, Timmerman JM. Dendritic cells loaded with apoptotic antibody-coated tumor cells provide protective immunity against B-cell lymphoma in vivo. Blood. 2008;111:1504–1511. doi: 10.1182/blood-2007-03-080507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez V, Hartmann E, Ott G, Campo E, Rosenwald A. Pathogenesis of mantle-cell lymphoma: all oncogenic roads lead to dysregulation of cell cycle and DNA damage response pathways. J Clin Oncol. 2005;23:6364–6369. doi: 10.1200/JCO.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Amin HM, McDonnell TJ, Medeiros LJ, Rassidakis GZ, Leventaki V, O'Connor SL, et al. Characterization of 4 mantle cell lymphoma cell lines. Arch Pathol Lab Med. 2003;127:424–431. doi: 10.5858/2003-127-0424-COMCLC. [DOI] [PubMed] [Google Scholar]
- 15.Jares P, Colomer D, Campo E. Genetic and molecular pathogenesis of mantle cell lymphoma: perspectives for new targeted therapeutics. Nat Rev Cancer. 2007;7:750–762. doi: 10.1038/nrc2230. [DOI] [PubMed] [Google Scholar]
- 16.Klier M, Anastasov N, Hermann A, Meindl T, Angermeier D, Raffeld M, et al. Specific lentiviral shRNA-mediated knockdown of cyclin D1 in mantle cell lymphoma has minimal effects on cell survival and reveals a regulatory circuit with cyclin D2. Leukemia. 2008;22:2097–2105. doi: 10.1038/leu.2008.213. [DOI] [PubMed] [Google Scholar]
- 17.Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B, et al. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Methods. 1996;196:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 18.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anton D, Dabadghao S, Palucka K, Holm G, Yi Q. Generation of dendritic cells from peripheral blood adherent cells in medium with human serum. Scand J Immunol. 1998;47:116–121. doi: 10.1046/j.1365-3083.1998.00284.x. [DOI] [PubMed] [Google Scholar]
- 20.Nijman HW, Houbiers JG, Vierboom MP, van der Burg SH, Drijfhout JW, D'Amaro J, Kenemans P, et al. Identification of peptide sequences that potentially trigger HLA-A2.1-restricted cytotoxic T lymphocytes. Eur J Immunol. 1993;23:1215–1219. doi: 10.1002/eji.1830230603. [DOI] [PubMed] [Google Scholar]
- 21.Qian J, Xie J, Hong S, Yang J, Zhang L, Han X, et al. Dickkopf-1 (DKK1) is a widely expressed and potent tumor-associated antigen in multiple myeloma. Blood. 2007;110:1587–1594. doi: 10.1182/blood-2007-03-082529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian J, Wang S, Yang J, Xie J, Lin P, Freeman ME, et al. Targeting heat shock proteins for immunotherapy in multiple myeloma: generation of myeloma-specific CTLs using dendritic cells pulsed with tumor-derived gp96. Clin Cancer Res. 2005;11:8808–8815. doi: 10.1158/1078-0432.CCR-05-1553. [DOI] [PubMed] [Google Scholar]
- 23.Fulcher D, Wong S. Carboxyfluorescein succinimidyl ester-based proliferative assays for assessment of T cell function in the diagnostic laboratory. Immunol Cell Biol. 1999;77:559–564. doi: 10.1046/j.1440-1711.1999.00870.x. [DOI] [PubMed] [Google Scholar]
- 24.Yi Q, Bergenbrant S, Osterborg A, Osby E, Ostman R, Bjorkholm M, et al. T-cell stimulation induced by idiotypes on monoclonal immunoglobulins in patients with monoclonal gammopathies. Scand J Immunol. 1993;38:529–534. doi: 10.1111/j.1365-3083.1993.tb03236.x. [DOI] [PubMed] [Google Scholar]
- 25.Yi Q, Osterborg A, Bergenbrant S, Mellstedt H, Holm G, Lefvert AK. Idiotype-reactive T-cell subsets and tumor load in monoclonal gammopathies. Blood. 1995;86:3043–3049. [PubMed] [Google Scholar]
- 26.Tourdot S, Scardino A, Saloustrou E, Gross DA, Pascolo S, Cordopatis P, et al. A general strategy to enhance immunogenicity of low-affinity HLA-A2. 1-associated peptides: implication in the identification of cryptic tumor epitopes. Eur J Immunol. 2000;30:3411–3421. doi: 10.1002/1521-4141(2000012)30:12<3411::AID-IMMU3411>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 27.Chen JL, Dunbar PR, Gileadi U, Jager E, Gnjatic S, Nagata Y, et al. Identification of NY-ESO-1 peptide analogues capable of improved stimulation of tumor-reactive CTL. J Immunol. 2000;165:948–955. doi: 10.4049/jimmunol.165.2.948. [DOI] [PubMed] [Google Scholar]
- 28.Tsomides TJ, Walker BD, Eisen HN. An optimal viral peptide recognized by CD8+ T cells binds very tightly to the restricting class I major histocompatibility complex protein on intact cells but not to the purified class I protein. Proc Natl Acad Sci U S A. 1991;88:11276–11280. doi: 10.1073/pnas.88.24.11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyall J, Latouche JB, Schnell S, Sadelain M. Lentivirus-transduced human monocyte-derived dendritic cells efficiently stimulate antigen-specific cytotoxic T lymphocytes. Blood. 2001;97:114–121. doi: 10.1182/blood.v97.1.114. [DOI] [PubMed] [Google Scholar]
- 30.de Jong R, Brouwer M, Miedema F, van Lier RA. Human CD8+ T lymphocytes can be divided into CD45RA+ and CD45RO+ cells with different requirements for activation and differentiation. J Immunol. 1991;146:2088–2094. [PubMed] [Google Scholar]
- 31.Cobb BS, Hertweck A, Smith J, O'Connor E, Graf D, Cook T, et al. A role for Dicer in immune regulation. J Exp Med. 2006;203:2519–2527. doi: 10.1084/jem.20061692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodland DL, Dutton RW. Heterogeneity of CD4(+) and CD8(+) T cells. Curr Opin Immunol. 2003;15:336–342. doi: 10.1016/s0952-7915(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 33.Pulendran B, Dillon S, Joseph C, Curiel T, Banchereau J, Mohamadzadeh M. Dendritic cells generated in the presence of GM-CSF plus IL-15 prime potent CD8+ Tc1 responses in vivo. Eur J Immunol. 2004;34:66–73. doi: 10.1002/eji.200324567. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Renkvist N, Sun Z, Schuler-Thurner B, Glaichenhaus N, Schuler G, et al. A polyclonal anti-vaccine CD4 T cell response detected with HLA-DP4 multimers in a melanoma patient vaccinated with MAGE-3.DP4-peptide-pulsed dendritic cells. Eur J Immunol. 2005;35:1066–1075. doi: 10.1002/eji.200425847. [DOI] [PubMed] [Google Scholar]
- 35.Berzofsky JA, Terabe M, Oh S, Belyakov IM, Ahlers JD, Janik JE, et al. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Invest. 2004;113:1515–1525. doi: 10.1172/JCI21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673–679. doi: 10.1016/s1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 37.Vonderheide RH, Anderson KS, Hahn WC, Butler MO, Schultze JL, Nadler LM. Characterization of HLA-A3-restricted cytotoxic T lymphocytes reactive against the widely expressed tumor antigen telomerase. Clin Cancer Res. 2001;7:3343–3348. [PubMed] [Google Scholar]
- 38.Schmitz M, Diestelkoetter P, Weigle B, Schmachtenberg F, Stevanovic S, Ockert D, et al. Generation of survivin-specific CD8+ T effector cells by dendritic cells pulsed with protein or selected peptides. Cancer Res. 2000;60:4845–4849. [PubMed] [Google Scholar]
- 39.Andersen MH, Pedersen LO, Becker JC, Straten PT. Identification of a cytotoxic T lymphocyte response to the apoptosis inhibitor protein survivin in cancer patients. Cancer Res. 2001;61:869–872. [PubMed] [Google Scholar]
- 40.Pisarev V, Yu B, Salup R, Sherman S, Altieri DC, Gabrilovich DI. Full-length dominant-negative survivin for cancer immunotherapy. Clin Cancer Res. 2003;9:6523–6533. [PubMed] [Google Scholar]
- 41.Gross DA, Graff-Dubois S, Opolon P, Cornet S, Alves P, Bennaceur-Griscelli A, et al. High vaccination efficiency of low-affinity epitopes in antitumor immunotherapy. J Clin Invest. 2004b;113:425–433. doi: 10.1172/JCI19418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuriyama H, Shimizu K, Lee W, Kjaergaard J, Parkhurst MR, Cohen PA, et al. Therapeutic vaccine generated by electrofusion of dendritic cells and tumour cells. Dev Biol (Basel) 2004;116:169–178. discussion 179–186. [PubMed] [Google Scholar]
- 43.Otto K, Andersen MH, Eggert A, Keikavoussi P, Pedersen LO, Rath JC, et al. Lack of toxicity of therapy-induced T cell responses against the universal tumour antigen survivin. Vaccine. 2005;23:884–889. doi: 10.1016/j.vaccine.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Marzec M, Kasprzycka M, Lai R, Gladden AB, Wlodarski P, Tomczak E, et al. Mantle cell lymphoma cells express predominantly cyclin D1a isoform and are highly sensitive to selective inhibition of CDK4 kinase activity. Blood. 2006;108:1744–1750. doi: 10.1182/blood-2006-04-016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Witzig TE. Current treatment approaches for mantle-cell lymphoma. J Clin Oncol. 2005;23:6409–6414. doi: 10.1200/JCO.2005.55.017. [DOI] [PubMed] [Google Scholar]
- 46.Quelle DE, Ashmun RA, Shurtleff SA, Kato JY, Bar-Sagi D, Roussel MF, et al. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 47.Lu XL, Jiang XB, Liu RE, Zhang FC, Zhao HY. Generation of allo-restricted cytotoxic T lymphocytes against malignant glioma by artificial antigen-presenting cells. Cancer Lett. 2007;256:128–135. doi: 10.1016/j.canlet.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 48.Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998;92:1927–1932. [PubMed] [Google Scholar]
- 49.McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]