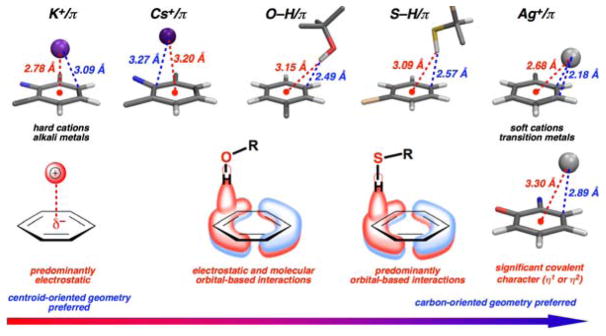
Figure 12.
Crystallographically observed representative geometries of cation/π, O–H/π, and S–H/π interactions. Interaction geometries represent a continuum of electrostatic and molecular orbital (covalent)-based effects. Classical (alkali metal) cation/π interactions are centroid-directed (η6-like) with geometries dictated by electrostatic effects. In contrast, transition metal cation/π interactions often exhibit substantial covalent character (η1-, η2-, or η3-interactions), in addition to electrostatic effects. O–H/π and S–H/π interactions similarly exhibit a combination of electrostatic and molecular orbital-based (covalent-like) effects, with a greater likelihood for location near the carbons (π molecular orbitals) and for H...C distances below the sum of the van der Waals radii than for K+/π interactions. Structures from left to right (CSD codes): YOLDAG, VIKDOK, AHOSAT, KOYJIT, PUXNUR, TITKUE; atoms not involved in the aromatic interaction have been removed for clarity.23h, 47 An analysis of Ag+/π interactions in the CSD is included in the Supporting Information