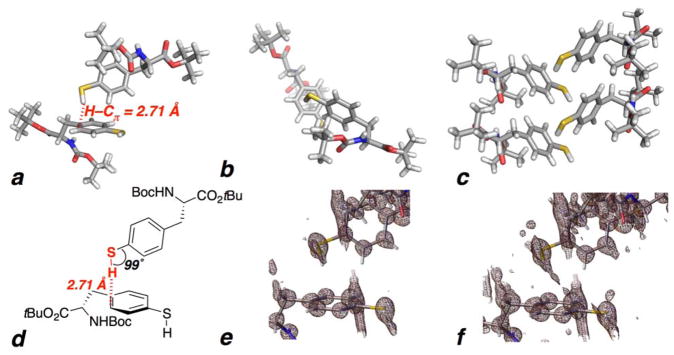
Figure 2.
(a–f) Crystal structure of Boc-L-4-thiolphenylalanine tert-butyl ester (1). The unit cell consists of one molecule of 1. (a,b,d) Two adjacent molecules of 1, highlighting the interaction of the thiol S–H with the adjacent aromatic ring, with a 2.71 Å Hthiol–Caromatic distance between the thiol hydrogen and the nearest aromatic carbon (below the 2.90 Å sum of the van der Waals radii). This electron density-based calculated Hthiol–Caromatic distance is based on a S–H bond length of 1.26 ± 0.04 Å, which is within the range of typical thiol S–H bond lengths (average 1.338 Å). The Hthiol-centroid distance is 2.90 Å. Gilli and Gilli noted that x-ray diffraction studies typically underestimate X–H bond lengths by 0.1–0.2 Å compared to data from neutron diffraction.11 If a standardized 1.338 Å S–H bond length is assigned to account for this potential systematic underestimation, as suggested by Gilli and Gilli, then the Hthiol–Caromatic distance would be 2.63 Å. The top view (b) shows the vector of the S–H bond directed toward the carbons of the aromatic ring. (c) The S–H/π interaction propagates through the crystal structure. No traditional hydrogen bond is observed to the thiol in the structure. The crystal packing is also mediated by an intermolecular hydrogen bond between the Boc carbamate NH and the carbamate C=O in an adjacent molecule. An intermolecular Haromatic-Caromatic interaction is also observed via the aromatic hydrogen adjacent to the thiol (2.84 Å). (e) Electron density map exhibiting the electron density associated with the thiol S–H bond. (f) Electron density map at greater contours showing electron density between the S–H bond and the aromatic ring. In contrast to 1, the crystal structure of p-thiocresol exhibits significant disorder, without ordering at the thiol, likely due to its more planar overall structure.41