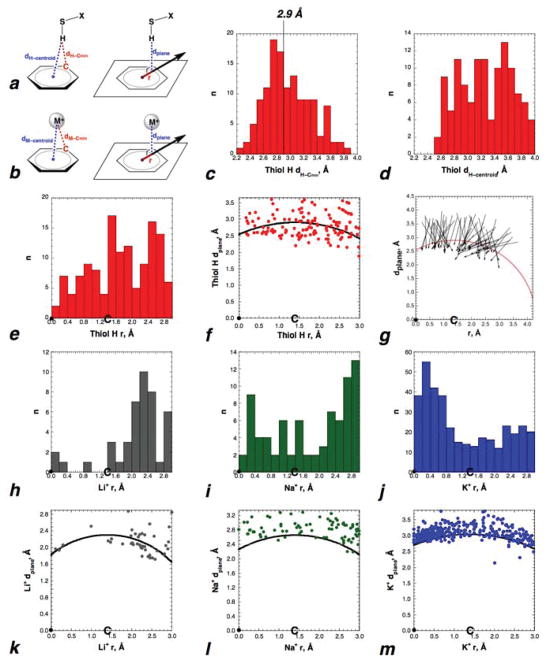
Figure 9.
Analysis of S–H/π interactions and alkali cation/π interactions, geometries, and distances in the Cambridge Structural Database (CSD). (a) Definitions of distances measured in S–H/π interactions: dH–Cmin = distance between the thiol H and the nearest aromatic carbon (shortest Hthiol–Caromatic distance); dH–centroid = distance between the thiol H and the centroid of the aromatic ring; dplane = distance from the thiol H to the plane of the ring, via projection at 90˚ to the plane of the ring; r = distance of the thiol H, projected onto the plane of the ring, from the centroid of the ring. • indicates the location of the centroid of the aromatic ring. C indicates the location of the carbons of the aromatic ring (1.40 Å from the centroid). Similar definitions were applied to the distances of the thiol sulfur (Supporting Information) and the analyzed cations from the ring and ring components. Full data sets from CSD searches are in Tables S1–S5. (b) Definition of distances in cation/π interactions. Definitions are as in (a), with M = Li+, Na+, or K+. Search parameters for (a) and (b) include all S–H and M+ with r ≤ 3.0 Å and with dplane ≤ 1.25 × Σ (van der Waals and/or ionic radii of C and H or M+) (c, d, e) Histograms of dH–Cmin, dH–centroid, and r for all thiols in this analysis. In contrast to expectations for a purely electrostatic interaction, hydrogens are on average closer to the carbons of the ring than to the centroid, and a significant percentage of the hydrogens are within the 2.9 Å sum of the van der Waals radii of H and C. (f) Comparison of dplane as a function of distance from the centroid of the ring. The 2.90 Å sum of the C and H van der Waals radii, indicated by the line (a radius of 2.90 Å centered on the aromatic C, which is 1.40 Å from the centroid of the ring), results in shorter “allowed” distances at the centroid of the ring than directly over the carbons of the ring. The centroid of the aromatic ring thus has two geometric advantages for a purely electrostatic interaction: the ability to interact with the entire electron cloud of the aromatic ring, and the ability to have a closer interaction with the ring without repulsive interactions. Despite these advantages, there are few thiol hydrogens near the centroid of the ring. (g) S→H bond vectors for all thiols in which dH–Cmin was ≤ 2.90 Å. Analysis in (c–g) was based on the S–H bond lengths in the CIF files (mean S–H bond length 1.20 ± 0.12 Å; mean X–S–H bond angle 102.8˚ ± 8.2˚; median X–S–H bond angle 100.3˚). Full analysis of thiols in the CSD with all S–H bond lengths normalized to 1.338 Å is in the Supporting Information (Table S2, Figures S104–S105, S107). Additional analysis is in the Supporting Information. (h–m) Li+, Na+, and K+ cation/π interactions in the CSD as a function of distance from the centroid (r) and distance from the plane (dplane). The line indicates the sum of the van der Waals and ionic radii of C and the indicated cation. K+ interactions in the CSD closely resemble the classical description of a cation/π interaction, with a preference for location of the cation near the centroid of the aromatic and distances at or somewhat greater than the sum of the van der Waals and ionic radii.