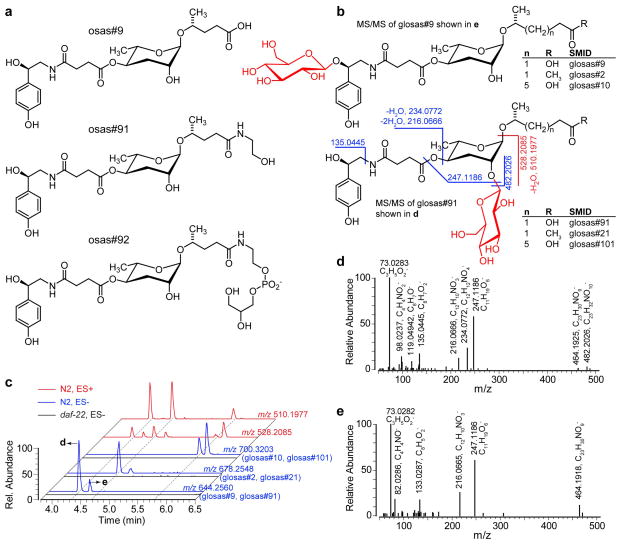
Figure 3.
a) Structures of osas#9 and examples of newly identified derivatives. b) Structures of two series of osas glucoside isomers with selected MS/MS fragmentations in ES− (blue) and ES+ (red) that distinguish isomers. c) EICs of osas glucosides. EICs in ES− (blue) correspond to glucosides of osas#9 (m/z 644.2560), osas#2 (m/z 678.2548) and osas#10 (m/z 700.3203). Neutral loss of fatty acid side chain in ES+ results in fragments common to all three osas glucosides (m/z 528.2085 for all glucosides and m/z 510.1977 after additional water loss, which is favored when the octopamine OH group is unsubstituted). d, e) MS/MS spectra of osas#9 glucosides in ES−. Absence of ions with m/z 135.0445, 234.0772, 482.2026 in e) suggests glycosylation of the octopamine hydroxy group in this isomer.