Abstract
Objective
Evidence suggests that social skills training (SST) is an efficacious intervention for negative symptoms in psychosis, whereas evidence of efficacy in other psychosis symptom domains is limited. The current article reports a comprehensive meta-analytic review of the evidence for SST across relevant outcome measures, control comparisons, and follow-up assessments. The secondary aim of this study was to identify and investigate the efficacy of SST subtypes.
Methods
A systematic literature search identified 27 randomized controlled trials including N = 1437 participants. Trials assessing SST against active controls, treatment-as-usual (TAU), and waiting list control were included. Risk of bias was assessed using the Cochrane risk of bias assessment tool. A series of 70 meta-analytic comparisons provided effect sizes in Hedges’ g. Heterogeneity and publication bias were assessed.
Results
SST demonstrated superiority over TAU (g = 0.3), active controls (g = 0.2–0.3), and comparators pooled (g = 0.2–0.3) for negative symptoms, and over TAU (g = 0.4) and comparators pooled (g = 0.3) for general psychopathology. Superiority was indicated in a proportion of comparisons for all symptoms pooled and social outcome measures. SST subtype comparisons were underpowered, although social-cognitive approaches demonstrated superiority vs comparators pooled. SST treatment effects were maintained at proportion of follow-up comparisons.
Conclusions
SST demonstrates a magnitude of effect for negative symptoms similar to those commonly reported for cognitive-behavioral therapy (CBT) for positive symptoms, although unlike CBT, SST is not routinely recommended in treatment guidelines for psychological intervention. SST may have potential for wider implementation. Further stringent effectiveness research alongside wider pilot implementation of SST in community mental health teams is warranted.
Keywords: social skills training, psychosis, schizophrenia, meta-analysis
Introduction
Social skills training (SST) is a psychological intervention focused upon the development or improvement of social interaction, social performance, or interpersonal skills, primarily offered to patients diagnosed with schizophrenia-spectrum disorders or psychosis. SST was initially developed in the context of the deinstitutionalization of psychiatric patients returning to the community in the 1970s and utilized behavioral techniques such as role-play, modeling, coaching, instruction, and feedback in an attempt to address interpersonal deficits. The literature from this period described SST as an effective means of reducing social anxiety, although suggested that improved generalizability to real-life situations was desirable.1
Since an initial wave of development in the 1980s and 1990s, SST has diversified meaning that a range of related interventions may now be subsumed within the terminology. The term SST, therefore, represents a broader spectrum of related interventions within the contemporary literature. These include approaches focused primarily on social cognition that may also integrate technology. Such approaches differ from the similar cognitive remediation methodology by their focus primarily upon social cognitive process and social perception rather than upon improving neuropsychological variables such as memory, attention, or executive function.2,3 Similarly, a number of SST approaches assimilate cognitive-behavioral techniques such as cognitive restructuring, although they follow an SST-style group format as opposed to the typical formulation-based approach of cognitive-behavioral therapy (CBT).4 Finally, a number of practically focused approaches integrating SST with psycho-education, life management skills, and relapse prevention strategies also exist.5,6
Negative symptoms refer to a specific pattern of commonly observed deficits in psychosis such as passive or apathetic social withdrawal, communication difficulties, blunting of affect, and rigid or stereotypical thinking.7 Comparatively less research has focused upon the treatment of negative symptoms than positive symptoms while fewer targeted interventions have been developed. Only in recent years have negative symptoms been included as primary outcomes in SST-based interventions since early studies focused on social functioning outcomes.1Fusar-Poli et al8 assessed the efficacy of pharmacological and psychological interventions for negative symptoms in a large meta-analysis and reported a medium effect size for second-generation antipsychotics vs placebo (g = 0.6, P ≤ .05), whereas their comparison of 10 randomised controlled trial (RCTs) for first-generation antipsychotics vs placebo was not significant (g = 0.05, P = .69). Both comparisons displayed a high degree of heterogeneity, whereas for psychological interventions pooled, they reported a small-to-medium effect size (g = 0.4, P ≤ .05) and moderate heterogeneity. The effect size for antidepressants was smaller (g = 0.3, P ≤ .05). The question of whether medication is more efficacious than psychological interventions pooled is not straightforward because the majority of participants in RCTs for psychological interventions are already maintained on antipsychotic medication which has impact upon target symptoms. However, this meta-analytic evidence suggests that differences in efficacy between psychological and pharmacological interventions for negative symptoms are small.8
A recent meta-analysis reported similar small-to-medium effect sizes (g = 0.3–0.6) in favor of SST when compared to other psychological interventions for negative symptoms in psychosis.9 Interestingly, the magnitude of the effect size increased with progressive sensitivity analyses to address risk of bias suggesting robustness. The UK National Institute for Health and Care Excellence (NICE) guidelines state that SST should not be offered as a specific intervention for psychosis following their conclusion in 2009 that SST did not show sufficient superiority over standard care alongside concerns regarding limited generalizability to everyday living,10 whereas in the United States, guidelines have suggested that SST is not an effective means to reduce symptoms.11 SST is not routinely integrated within adult clinical psychology or community mental health settings in the UK National Health Service (NHS). CBT is the most widely recommended and integrated psychological intervention for psychosis in the UK, although many CBT manuals focus primarily on addressing positive rather than negative symptoms of psychosis.12 Earlier meta-analytic evidence suggested that CBT may be effective for negative symptoms (g = 0.4, P < .05).13 This effect was not, however, maintained when the authors excluded nonrandomized studies and could not be replicated in a more recent meta-analysis when negative symptoms were primary (g = 0.2, P > .05) or secondary (g = 0.1, P > .05) outcomes.14 The consideration that SST appears relatively more efficacious than CBT in reducing negative symptoms and has produced effect sizes comparable to pharmacological treatments suggests that further examination of its clinical utility is warranted.
The current review aimed to expand upon the promising meta-analytic evidence for SST from our previous comparative meta-analysis of psychological interventions for psychosis by applying a more comprehensive focus on SST and including all comparison conditions rather than only bona fide psychological interventions. To the best of our knowledge, it is 8 years since SST has been thoroughly examined via meta-analysis.15 Given the accumulation of articles since this time means that a renewed evaluation of its effectiveness is warranted. Because SST has further diversified into a range of related interventions, we aimed to define and assess subtypes of SST as an adjunct to our primary comparisons. We also aimed to account for varying methodological rigor among SST trials because previous reviews did not address risk of bias within RCTs.16,17 Our overall aim was therefore to provide a detailed meta-analytic review of the contemporary evidence-base for SST, with robust appraisal of risk of bias and methodological quality in RCTs. Our primary objective was to determine whether SST and SST subtypes demonstrate superiority in reducing negative symptoms against relevant comparison conditions. We hypothesized that SST would demonstrate superiority for negative symptoms across comparisons, whereas superiority would not be demonstrated in other symptom domains.
Methods
A systematic literature search and meta-analysis was performed following PRISMA guidelines for the reporting of systematic reviews and meta-analyses.18
Protocol
The objectives and intended methodology of this project were registered via PROSPERO on May 9, 2016 and can be obtained at the following web location; http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016038872.
Search Strategy
A systematic literature search was completed in May 2016 (with no limits applied for year of publication) and included four databases: The Cochrane Central Register of Controlled Trials, Pubmed, PsychInfo, and Embase. Abstracts were identified by entering text variations of three key terms dependent upon Boolean operators, MeSH terms, exploded terms, and limit settings relevant to each database, namely, (1) social skills training and related interventions, (2) psychosis and related diagnoses, and (3) randomized controlled trials. Further search strings have been included in Supplementary Material. Articles included in published meta-analyses were also considered for inclusion.9,16,17,19,20 Trial registrations, conference abstracts, and dissertations were also considered via grey literature checks online.
Study Selection
Studies were included if they were randomized controlled trials in which social skills training or related interventions were compared against a control condition and applied to a psychosis population. Studies also met the following inclusion criteria: (a) the participants were diagnosed with psychotic disorders including schizophrenia, schizoaffective disorder, delusional disorder, brief psychotic disorder, or psychosis not otherwise specified (NOS); (b) the intervention was defined as SST or was primarily intended to improve social performance; (c) the study was fully randomized and included comparison to an active control, treatment-as-usual (TAU), or a waiting list control; and (d) relevant outcome measures assessing psychotic symptoms and/or social performance were reported at post-treatment and/or follow-up. Active controls include comparison of SST against other bona fide interventions such as cognitive-behavioral therapy and therefore provide the most stringent comparison.
Studies were excluded if (a) participants had alternative or comorbid diagnoses, such as substance abuse or ultra-high risk of psychosis; (b) missing data could not be obtained by contacting authors; or (c) authors mixed elements of SST and other interventions into the intervention and/or control condition resulting in difficulty comparing the active SST element (eg, SST plus oxytocin). Only studies reported in the English language were included.
Risk of Bias Assessment
For consistency with the previous meta-analysis,9 RCTs were assessed at the study level against the first four criteria of the Cochrane risk of bias tool: sequence generation, allocation concealment, blinding of assessors, and incomplete outcome data. The final two items (selective outcome reporting and other sources of bias) were omitted because there is no evidence of their impact upon validity in meta-analysis.21 The third item (blinding of assessors) was adapted to include only outcome assessors in blinding because, unlike medication trials, study therapists and participants cannot be blinded to the intervention being delivered. Two authors (D.T. and E.M.G.) calculated risk of bias scores via independent rating and resolution by discussion for 13 (48%) of the included studies, whereas risk of bias assessments for 14 (52%) studies were utilized from the previously published meta-analysis.9 Risk of bias items were rated as high-risk or low-risk, whereas unclear items were categorized as high-risk.
Data Extraction
Symptom-related outcome data were extracted from 14 studies as part of the previous publication.9 These data were checked for consistency and included in the current analysis. One author (D.T.) extracted symptom-related outcome data from the remaining 13 studies and extracted social performance outcome data for all studies, whereas another (E.M.G.) checked consistency. Spreadsheets piloted and utilized in the previous meta-analysis were employed for extraction. We contacted five authors22–25 with requests for missing or unpublished outcome data, resulting in one successful further inclusion.26
Outcome Measures
All continuous outcome measures relevant to psychotic symptoms, general psychopathology, and social performance were extracted. We considered negative symptoms the primary outcome measure based on results of the previous meta-analysis.9 In instances where multiple outcome measures were reported within one domain, all data were extracted and combined to form a pooled effect size for that domain. In a minority of studies, only dichotomous outcome data were available. These were converted into Hedges’ g according to the methods integrated in Comprehensive Meta-Analysis (CMA).27 The all symptoms comparison therefore includes relapse, discharge, and clinical exacerbation as proxy symptom measures.
Meta-Analyses
The overall strategy for the meta-analyses was to progress gradually from a broad and inclusive sample of studies toward more methodologically robust comparisons. This meant that for each outcome measure category (all symptoms, positive symptoms, negative symptoms, general symptoms, and social performance) or comparison category (all comparators, active controls, TAU, and supportive counseling [SC] only), separate meta-analyses were performed for progressively decreasing risk of bias (0–4, where 4 indicates the highest risk of bias) when possible based on study availability. Meta-analyses were performed on outcome measures or comparator categories when at least five studies were available. Risk of bias sensitivity analyses were performed when at least four studies were available. It was acknowledged that comparisons meeting the minimum required number of studies would be considerably underpowered.
In order to investigate differences in efficacy between SST variations and related interventions, two authors (D.T. and A.M.) identified subtypes of SST independently and resolved disagreements by discussion before final categorization. Separate meta-analyses were then performed using the same procedures as above. Similarly, meta-analyses for outcome measures assessed at follow-up were conducted when there were at least four studies available at any given follow-up time point (eg, 6 months).
For meta-analyses which did not require the combination of outcome measures at study level, the computer software R Studio version 1.0.136 was used to calculate pooled effect sizes using the packages meta and metafor.28,29 For comparisons that included studies where two outcome measures were reported in the same domain (eg, two measures of negative symptoms), Comprehensive Meta-Analysis, version 3.0 was used due to its ability to provide a combined effect size at the study level. The programs were checked for consistency of results on a proportion of comparisons. Both software packages provided an aggregated effect size indicating the pooled mean difference between groups at post-treatment or follow-up using Hedges’ g. Hedges’ g is an estimate of the standardized mean difference between groups and provides a more accurate estimate of effects in small samples than similar statistics for continuous outcome variables such as Cohen’s d.30 Alpha was set to 0.05 for all comparisons and 95% confidence intervals were obtained.
Heterogeneity
Both software packages calculated chi-square tests to assess the degree of heterogeneity for each comparison. The Q statistic and resultant alpha level were used to determine the presence of heterogeneity in each comparison. The I2 statistic described the percentage of variance in each comparison that may arise from heterogeneity between studies or outcome measures rather than by chance. For the purpose of assessment, heterogeneity was defined as absent (0%), low (25%), moderate (50%), and high (75%).31 A 95% confidence interval was calculated for the I2 statistic.
Publication Bias
Publication bias for all meta-analyses was established by examining funnel plots.32 Duval and Tweedie’s33 trim and fill procedure was used to estimate effect sizes after accounting for publication bias, whereas Egger’s34 test of the intercept was applied to quantify bias and assess significance.
Power Analysis
Due to progressive sensitivity analyses and our identification of SST subtypes, a number of comparisons were likely to be underpowered. We therefore utilized power analysis to determine the approximate number of studies required to identify relevant effects. Previous meta-analysis identified effect sizes ranging from roughly g = 0.2–0.6 for SST.9 Based on Cuijpers’35 table, for an average N of 30 per group in each study and conservatively assuming 0.80 power alongside alpha level 0.05, it was estimated that 18 studies would be required to detect an effect size of g = 0.2 for comparisons with low between study variances. Comparisons with medium and high variances would require 22 and 26 studies, respectively.
Results
Study Selection
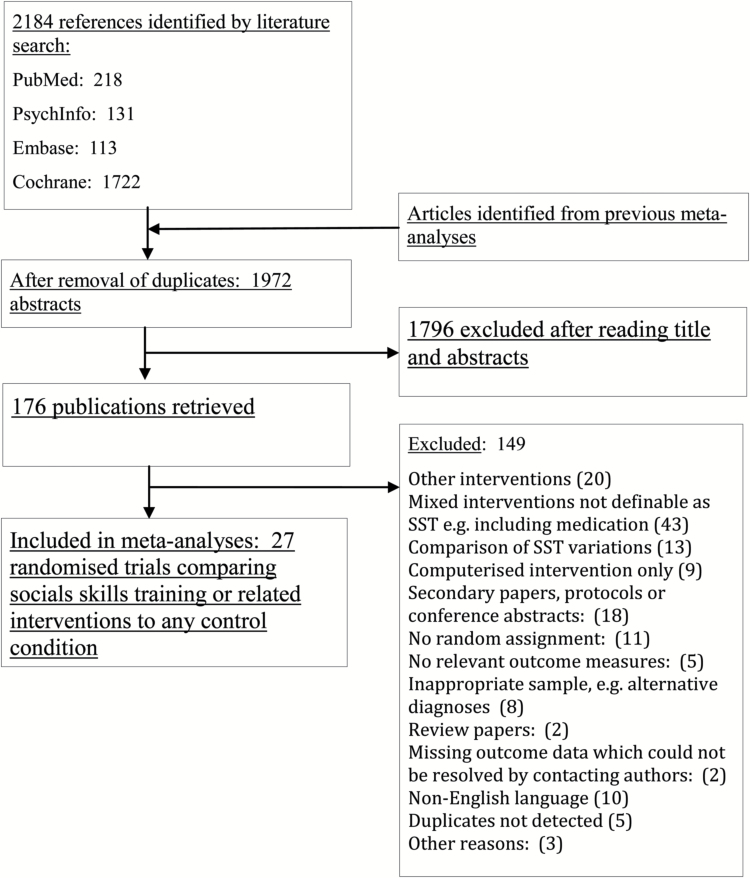
Figure 1 illustrates the selection process by which articles were screened for inclusion. Following removal of duplicates, 1972 title abstracts were screened for relevant characteristics; a further 176 articles were retrieved for closer inspection of inclusion and exclusion criteria. Twenty-seven randomized controlled trials qualified for final inclusion resulting in data for N = 1437 participants being included across 70 meta-analyses and sensitivity analyses. All included RCTs reported outcome measures at post-treatment, whereas 11 studies (40%) included follow-up data ranging from 12 weeks to 18 months post-treatment.
Fig. 1.
PRISMA flowchart of inclusion of studies.
Selected characteristics of included studies are available in Table 1. Twenty-five studies (93%) applied group format, whereas only two applied individual format. Risk of bias scores within studies ranged from 1 to 4. This meant that no studies achieved the lowest possible risk of bias score, and therefore, sensitivity analyses could not exclude all risk of bias. Details of risk of bias assessments at the study level are included in Supplementary Material. Four broad subtypes of SST were identified as defined in Table 2 and formed the basis of subtype comparisons.
Table 1.
Selected Characteristics of Included Randomized Controlled Trials of Social Skills Training and Related Interventions
| Study and publications | Country | Sample characteristics | Relevant comparisons and N | Symptom outcome measures | Format | Bias risk (0–4) | Duration (wk to PT approximately) | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Anzai et al24 | Japan | DSM-IV and ICD- 10 schizophrenia. Inpatients. Refractor. Poor insight | SST (37) vs OT (15) | Rehab scale, Discharge | Group | 4 | 9 | N/A |
| Bowie et al51 | Canada and USA | Schizophrenia or schizoaffective disorder. Outpatients | SST (38) vs CR (38) | PANSS, SSPA | Group | 1 | 12 | 12 wk |
| Chien et al52 | Taiwan | DSM-IV schizophrenia. Inpatients | SST (35) vs TAU (43) | PANSS, IAS | Group | 3 | 4 | N/A |
| Choi et al53 | South Korea | DSM-IV schizophrenia and schizo-affective disorder. Outpatients | SST (17) vs TAU (17) | SBST | Group | 4 | 26 | N/A |
| Dobson et al54 | Canada | DSM-III Schizophrenia. Outpatients. Severe patients excluded | SST (15) vs BF 13) | PANSS | Group | 3 | 11 | N/A |
| Gohar et al2 | Egypt | DSM-IV schizophrenia and schizo-affective disorder. Outpatients | SCST (22) vs CST (20) | PANSS, MSCEIT | Group | 3 | 8 | N/A |
| Granholm et al55,56 | USA | DSM-IV schizophrenia and schizo-affective disorder. Older outpatients 42+ | CBSST (37) vs TAU (39) | PANSS | Group | 2 | 24 | 6, 12 mo |
| Granholm et al57 | USA | Older outpatients 45+, DSM-IV schizophrenia, and schizoaffective disorder | CBSST (41) vs SC (38) | PANSS, SANS | Group | 1 | 36 | 4.5, 9 mo |
| Granholm et al4 | USA | DSM-IV schizophrenia and schizoaffective disorder. Outpatients | CBSST (73) vs SC (76) | PANSS, SANS, MASC | Group | 1 | 36 | 6, 12 mo |
| Hayes et al58 | Australia | DSM-III-R schizophrenia. Non-current positive symptoms. Recruited from a range of services | SST (23) vs. SC (22) | BPRS, SANS, SSIT | Group | 4 | 18 | 6 mo |
| Hogarty et al59,60 | USA | RDC schizophrenia or schizoaffective disorder. High expressed emotion families. Inpatients | SST (23) vs FI (23) | Symptom relapse | Individual | 4 | 104 | N/A |
| Horan et al3 | USA | DSM-IV schizophrenia or schizoaffective disorder. Clinically stable outpatients | SST (17) vs PE (17) | BPRS, SSPA | Group | 2 | 6 | N/A |
| Horan et al61 | USA | DSM-IV schizophrenia, schizoaffective disorder, delusional disorder or psychosis. Clinically stable outpatients. | SST (19) vs CR (24) | BPRS, SSPA, HAM-D | Group | 2 | 12 | N/A |
| Lecomte et al62,63 | Canada | Early psychosis (< 2 years). Current psychotic symptoms. Stabilized outpatients | CBT (48) vs SST (54) | BPRS | Group | 2 | 13 | 6, 12 mo |
| Liberman et al64–66 | USA | DSM-III schizophrenia. Inpatients | SST (14) vs PE (14) | PAS | Group | 3 | 10 | N/A |
| Study and publications | Country | Sample characteristics | Relevant comparisons and N | Extracted outcome measures | Format | Bias Risk (0–4) | Duration (weeks to PT) | Follow-up |
| Liberman et al67 | USA | Persistent and unremitting schizophrenia. Outpatients | SST (42) vs OT (42) | BSI, GAS, BPRS | Both | 3 | 26 | N/A |
| Marder et al68 | USA | DSM-III schizophrenia. At least two acute episodes or 2 y psychotic symptoms. Male outpatients | SST (13) vs SC (14) | BPRS Exacerbations | Group | 3 | 104 | N/A |
| Ng et al69 | Hong Kong | DSM-IV schizophrenia. Inpatients | SST (18) vs SC (18) | BPRS, SANS, SFS, SBS | Group | 0 | 8 | 6 mo |
| Patterson et al70 | USA | DSM-IV schizophrenia or schizophreniform disorder. Older chronic Latino inpatients | SST (21) vs SC (8) | PANSS, SSPA | Group | 3 | 26 | 12, 18 mo |
| Patterson et al5 | USA | DSM-IV schizophrenia or schizophreniform. Older chronic inpatients | SST (124) vs SC (116) | PANSS, SSPA, HAM-D | Group | 2 | 26 | N/A |
| Roberts et al50 | USA | DSM-IV schizophrenia or schizoaffective disorder. Interaction difficulties. Outpatients | SCIT (33) vs. TAU (33) | PANSS, SSPA, GSFS | Group | 2 | 13 | 3 mo |
| Rus-Calafell et al71 | Spain | DSM-IV-TR schizophrenia or schizoaffective disorder. Clinically stable outpatients | SST (13) vs TAU (18) | PANSS, SFS | Group | 4 | 26 | 6 mo |
| Gil Sanz et al47 | Spain | CIE-10 Schizophrenia. Rehab patients | SCT (7) vs TAU (7) | PANSS, WHODAS-II | Group | 3 | 10 | N/A |
| Tas et al49 | Turkey and Germany | DSM-IV schizophrenia. Clinically stable outpatients | SST (22) vs BF (27) | PANSS, SFS | Group | 0 | 16 | N/A |
| Velligan et al26 | USA | DSM-IV Schizophrenia or schizoaffective disorder. Clinically stable outpatients | CBSST (26) vs TAU (25) | NSA-16, BNSS | Individual | 1 | 39 | N/A |
| Wang et al25 | China | DMS-IV schizophrenia. Clinically stable outpatients | SST (48) vs SC (48) | PSP | Group | 2 | 20 | N/A |
| Xiang et al6 | China | DSM-IV schizophrenia. Clinically stable inpatients and outpatients. | SST (50) vs PE (53) | PANSS, SDSS | Group | 1 | 4 | 6, 12 mo |
Note: BF, Befriending; BPRS, Brief Psychiatric Rating Scale; BNSS, Brief Negative Symptom Scale; BSI, Brief Symptom Inventory; CBT, Cognitive-Behavioral Therapy; CR, Cognitive Remediation; CST, Control Skills Training; FI, Family Intervention; GAS, Global Assessment Scale; GSFS, Global Social Functioning Scale; Ham-D, Hamilton Depression Rating Scale; IAS, Interaction Anxiety Scale; MASC, Maryland Assessment of Social Competence; MSCEIT, Mayer-Salovey Emotional Intelligence Test; N, Number of participants in each treatment group; NSA-16, Negative Symptoms Assessment; OT, Occupational Therapy; PANSS, Positive and Negative Syndromes Scale; PE, Psycho-education; PSP, Personal and Social Performance Scale; PT, Post-treatment; N/A, Not Applicable;,SANS, Scale for Assessment of Negative Symptoms; SBS, Social Behavior Schedule; SBST, Social Behavior Sequencing Task; SC, Supportive Counseling; SDSS, Social Disability Screening Schedule; SFS, Social Functioning Scale; SSPA, SSIT, Simulated Social Interaction Test; Social Skills Performance Assessment; SST, Social Skills Training; WHODAS-II, WHO Disability Scale.
Table 2.
Social Skills Training (SST) Subtype Descriptions and Comparison Types
| Definition | N st | N p |
|---|---|---|
| 1. Cognitive-behavioral social skills training (CBSST): CBSST defined interventions which utilized primarily a social-skills training approach similar to generic SST but also integrated cognitive-behavioral techniques such as cognitive restructuring, thought challenging or behavioral experiments. To limit heterogeneity, we attempted to exclude interventions that were primarily structured as formulation-based CBT-based approaches that added aspects of SST because these interventions have less explicit skills training focus.4,26 | 44,26,43,45 | 243 |
| 2. Generic social skills training: Generic SST refers to approaches that remain close to the original model of SST emerging in the 1980s. Typically this consists of a behaviorally oriented, group intervention based upon social learning traditions in which the therapist(s) engage participants in interpersonal training sessions. The focus is typically upon assertiveness, verbal and non-verbal communication, reduction of social distress, and learning appropriate contextual responses in social situations. This may be achieved via modeling, role-play, rehearsal, group reflection and discussion, or a variety of related methods.1,48 | 740,42,46,47,52,57,59 | 287 |
| 3. Social-cognitive skills training (SCST): This category refers to a relatively broad range of interventions that focus primarily on refining social cognitive processes such as emotion perception, theory-of-mind abilities. In order to qualify, interventions were required to include a therapist-led, behavioral, or reflective element in order to demonstrate distinction from approaches further on a continuum toward cognitive remediation. SCST may integrate computer programs or videos in order to facilitate improved training of social responses and may also follow a “drill and repeat” structure.49,50 | 82,3,25,37,38,41,49,60 | 295 |
| 4. UCLA-FAST based: The acronym for this category refers firstly to those interventions explicitly based upon the University of California Los Angeles (UCLA) model of skills training, which integrates traditional SST alongside aspects of psycho-education, relapse prevention, and skills in managing daily life tasks such as medication or independent travel. A similar approach is functional adaptive skills training (FAST); therefore, these varieties of SST were combined to form a more practical-skills based category.5,6,51 | 85,6,24,39,50,52,55,58 | 612 |
| 5. Treatment-as-usual (TAU) comparison: Refers to standard clinical care received by patients. TAU cannot be considered an active control in meta-analysis because intervention is nonstandardized, whereas both intervention and control groups in psychosis are likely to receive some form of TAU (eg, medication). | ||
| 6. Active controls: Includes bona-fide interventions such as cognitive-behavioral therapy alongside less recognized but standardized control interventions such as supportive counseling | ||
| 7. Supportive counseling (SC): Refers to nondirective supportive therapeutic contact which includes key common ingredients of therapy such as empathy and rapport without specific techniques of therapy models.39 |
Note: Nst, number of studies; Np, number of participants who received each intervention.
Effect of SST on Psychosis Symptoms
Results for all comparisons of SST against active controls, TAU, SC, and all comparators pooled are provided in Table 3. A summary forest plot of significant comparisons is provided in Figure 2. Separate meta-analyses were calculated for each symptom category and followed by risk of bias sensitivity analyses. SST was more efficacious than TAU for all symptoms (g = 0.28, P = .02) but did not demonstrate superiority against comparators pooled, active controls, or SC. The effect vs TAU was robust when removing studies with risk of bias scores of ≥4 (where 4 indicates the highest risk of bias score), although further sensitivity analyses were not possible due to limited study availability and the significant ≥4 comparison was underpowered. Heterogeneity was absent in the TAU comparison, although other nonsignificant comparisons for all symptoms pooled showed moderate-to-high heterogeneity. SST did not demonstrate superiority in any comparison for positive symptoms while heterogeneity was also moderate to high in this domain.
Table 3.
Effect Sizes of SST Across Outcome Measures and Comparison Conditions
| N | G | 95% CI | Z | P of Z | Q-value | I 2 (%) | I 2 95% CI | |
|---|---|---|---|---|---|---|---|---|
| SST for all symptom measures pooled | ||||||||
| Vs any comparator | ||||||||
| All eligible studies | 25 | 0.097 | −0.074, 0.267 | 1.112 | .266 | 53.99* | 55.48 | 30–72 |
| Excluding risk of bias score of 4 | 21 | 0.090 | −0.091, 0.270 | 0.973 | .331 | 46.13* | 56.64 | 29–73 |
| Excluding risk of bias score ≥3 | 13 | 0.106 | −0.131, 0.343 | 0.879 | .379 | 34.59* | 65.30 | 37–81 |
| Excluding risk of bias score ≥2 | 7 | 0.173** | −0.026, 0.373 | 1.704 | .088 | 6.42 | 6.49 | 0–73 |
| Vs active controls | ||||||||
| All eligible studies | 18 | 0.067 | −0.151, 0.286 | 0.605 | .545 | 45.23* | 62.42 | 37–77 |
| Excluding risk of bias score of 4 | 15 | 0.051 | −0.118, 0.281 | 0.440 | .660 | 37.30* | 62.47 | 34–79 |
| Excluding risk of bias score ≥3 | 10 | 0.088 | −0.209, 0.385 | 0.581 | .561 | 32.38* | 72.20 | 47–85 |
| Excluding risk of bias score ≥2 | 6 | 0.165 | −0.061, 0.392 | 1.431 | .152 | 6.19 | 19.15 | 0–64 |
| Vs TAU | ||||||||
| All eligible studies | 6 | 0.282* | 0.049, 0.515 | 2.373 | .018 | 2.80 | 0.00 | 0–75 |
| Excluding risk of bias score of 4 | 5 | 0.300* | 0.054, 0.546 | 2.386 | .017 | 2.61 | 0.00 | 0–79 |
| Vs SC only | ||||||||
| All eligible studies | 7 | −0.104 | −0.456, 0.247 | −0.58 | .560 | 18.88* | 68.23 | 30–86 |
| Excluding risk of bias score of 4 | 6 | −0.090 | −0.499, 0.318 | −0.432 | .666 | 18.81* | 73.42 | 39–88 |
| Excluding risk of bias score ≥3 | 4 | −0.086 | −0.669, 0.488 | −0.294 | .769 | 17.99* | 83.32 | 58–93 |
| SST for positive symptoms | ||||||||
| Vs any comparator | ||||||||
| All eligible studies | 13 | 0.0895 | −0.117, 0.296 | 0.85 | .397 | 23.88* | 49.8 | 5–73 |
| Excluding risk of bias score of 4 | 12 | 0.984 | −0.122, 0.318 | 0.88 | .381 | 23.72* | 53.6 | 11–76 |
| Excluding risk of bias score ≥3 | 9 | 0.980 | −0.150, 0.350 | 0.78 | .438 | 18.36* | 56.4 | 8–79 |
| Excluding risk of bias score ≥2 | 5 | 0.050 | −0.362, 0.460, | 0.23 | .819 | 14.70* | 72.8 | 32–89 |
| Vs active controls | ||||||||
| All eligible studies/ excluding risk of bias 4 | 8 | 0.080 | −0.223, 0.380, | 0.50 | .620 | 19.80* | 64.6 | 25–83 |
| Excluding risk of bias score ≥3 | 7 | 0.127 | −0.194, 0.450, | 0.78 | .437 | 18.04* | 66.7 | 26–85 |
| Excluding risk of bias score ≥2 | 5 | 0.050 | −0.362, 0.460, | 0.23 | .819 | 14.70* | 72.8 | 32–89 |
| Vs TAU | ||||||||
| All eligible studies | 5 | 0.151 | −0.098, 0.400, | 1.19 | .235 | 3.68 | 0.00 | 0–79 |
| Excluding risk of bias score of 4 | 4 | 0.176 | −0.110, 0.460, | 1.22 | .222 | 3.31 | 9.30 | 0–86 |
| SST for negative symptoms | ||||||||
| Vs any comparator | ||||||||
| All eligible studies | 17 | 0.191* | 0.043, 0.338 | 2.53 | .011 | 19.67 | 18.65 | 0–54 |
| Excluding risk of bias score of 4 | 15 | 0.218* | 0.077, 0.359 | 3.03 | .002 | 14.66 | 4.48 | 0–56 |
| Excluding risk of bias score ≥3 | 11 | 0.194* | 0.041, 0.346 | 2.49 | .013 | 7.96 | 0.00 | 0–60 |
| Excluding risk of bias score ≥2 | 7 | 0.279* | 0.087, 0.471 | 2.85 | .004 | 5.07 | 0.00 | 0–71 |
| Vs active controls | ||||||||
| All eligible studies | 11 | 0.136 | −0.070, 0.341 | 1.29 | .196 | 16.01 | 37.52 | 0–69 |
| Excluding risk of bias score of 4 | 10 | 0.185** | −0.009, 0.378 | 1.87 | .061 | 11.94 | 24.61 | 0–64 |
| Excluding risk of bias score ≥3 | 8 | 0.196* | 0.010, 0.383 | 2.07 | .039 | 0.74 | 9.54 | 0–68 |
| Excluding risk of bias score ≥2 | 6 | 0.276* | 0.073, 0.478 | 2.67 | .008 | 5.05 | 1.04 | 0–75 |
| Vs TAU | ||||||||
| All eligible studies | 6 | 0.311* | 0.078, 0.544 | 2.61 | .009 | 2.17 | 0.00 | 0–75 |
| Excluding risk of bias score of 4 | 5 | 0.300* | 0.054, 0.546 | 2.39 | .017 | 2.09 | 0.00 | 0–79 |
| Vs SC only | ||||||||
| All eligible studies | 4 | 0.013 | −0.283, 0.257 | 0.09 | .927 | 2.77 | 0.00 | 0–85 |
| SST for PANSS general symptoms | ||||||||
| Vs any comparator, all eligible studies | 6 | 0.318* | 0.043, 0.594 | 2.26 | .023 | 7.33 | 31.70 | 0–72 |
| Vs TAU, all eligible studies | 4 | 0.404* | 0.111, 0.697 | 2.70 | .007 | 2.31 | 0.00 | 0–85 |
| SST for social competency outcome measures | ||||||||
| Vs any comparator | ||||||||
| All eligible studies | 17 | 0.326* | 0.079, 0.572 | 2.59 | .010 | 49.60* | 67.79 | 47–81 |
| Excluding risk of bias score of 4 | 13 | 0.364* | 0.100, 0.628 | 2.70 | .007 | 37.27* | 67.80 | 43–82 |
| Excluding risk of bias score ≥3 | 9 | 0.372* | 0.036, 0.709 | 2.17 | .030 | 33.20* | 75.91 | 54–87 |
| Excluding risk of bias score ≥2 | 5 | 0.193 | −0.065, 0.451 | 1.47 | .143 | 5.44 | 26.48 | 0–72 |
| Vs active controls | ||||||||
| All eligible studies | 12 | 0.131 | 0.234, 0496 | 0.70 | .482 | 59.59* | 81.53 | 69–89 |
| Excluding risk of bias score of 4 | 10 | 0.227 | −0.170, 0.624 | 1.12 | .262 | 51.16* | 82.41 | 69–90 |
| Excluding risk of bias score ≥3 | 8 | 0.320 | −0.098, 0.738 | 1.50 | .134 | 39.98 | 82.49 | 67–91 |
| Excluding risk of bias score ≥2 | 5 | 0.020 | −0.312, 0.353 | 0.12 | .906 | 8.86 | 54.88 | 0–83 |
| Vs TAU | ||||||||
| All eligible studies | 5 | 0.201 | −0.140, 0.541 | 1.16 | .248 | 5.31 | 24.69 | 0–70 |
| SST subtypes vs any comparator | ||||||||
| All symptom measures pooled | ||||||||
| Generic SST, all eligible studies | 7 | 0.171 | −0.127, 0.468 | 1.13 | .260 | 8.70 | 31.77 | 0–71 |
| Generic SST, excl. risk of bias ≥4 | 4 | 0.364** | −0.011, 0.739 | 1.90 | .057 | 4.21 | 28.71 | 0–74 |
| Cognitive-behavioral SST, excl. risk of bias ≥3 | 4 | 0.147 | −0.108, 0.403 | 1.13 | .258 | 0.59 | 0.00 | 0–85 |
| Social-cognitive SST, excl. risk of bias ≥4 | 6 | 0.270 | −0.027, 0.567 | 1.78 | .075 | 6.40 | 21.92 | 0–66 |
| Social-cognitive SST, excl. risk of bias ≥3 | 5 | 0.392* | 0.107, 0.678 | 2.70 | .007 | 2.48 | 0.00 | 0–79 |
| Social-cognitive SST, excl. risk of bias ≥2 | 4 | 0.413* | 0.116, 0.709 | 2.73 | .006 | 2.24 | 0.00 | 0–85 |
| UCLA-FAST, all eligible studies | 8 | −0.058 | −0.392, 0.276 | −0.34 | .733 | 25.19* | 72.21 | 43–86 |
| UCLA-FAST, risk of bias ≥4 | 7 | −0.176 | −0.461, 0.109 | −1.21 | .226 | 15.71* | 61.81 | 13–83 |
| UCLA-FAST, excl. risk of bias ≥3 | 4 | −0.201 | −0.649, 0.246 | −0.88 | .378 | 14.65 | 79.52 | 46–92 |
| Negative symptoms | ||||||||
| Generic SST, all eligible studies | 5 | 0.268 | −0.143, 0.678 | 1.28 | .201 | 8.66 | 53.83 | 0–83 |
| Cognitive-behavioral SST, all eligible studies | 4 | 0.146 | −0.117, 0.402 | 1.11 | .266 | 0.46 | 0.00 | 0–85 |
| Social-cognitive SST, all eligible studies | 5 | 0.148 | −0.213, 0.509 | 0.80 | .421 | 6.47 | 38.14 | 0–77 |
| Social competency outcome measures | ||||||||
| Generic SST, all eligible studies | 4 | −0.031 | −0.318, 0.256 | 0.21 | .832 | 1.31 | 0.00 | 0–85 |
| Social-cognitive SST, all eligible studies | 7 | 0.301 | −0.211, 0.812 | 1.15 | .249 | 23.41* | 74.37 | 45–88 |
| Social-cognitive SST, excl. risk of bias ≥4 | 6 | 0.188 | −0.340, 0.716 | 0.70 | .485 | 19.86* | 74.82 | 43–89 |
| Social-cognitive SST, excl. risk of bias ≥3 | 4 | 0.478** | −0.018, 0.975 | 1.89 | .059 | 8.38* | 64.18 | 0–88 |
| UCLA-FAST, all eligible studies | 5 | 0.080 | −0.587, 0.747 | 0.24 | .814 | 36.19* | 88.95 | 77–95 |
| UCLA-FAST, excl. risk of bias ≥4 | 4 | 0.267 | −0.432, 0.966 | 0.75 | .454 | 27.9* | 89.25 | 75–95 |
| SST vs any comparator at 6 mo follow-up | ||||||||
| All symptoms, all eligible studies | 8 | 0.035 | −0.150, 0.220 | 0.37 | .712 | 1.94 | 0.00 | 0–68 |
| All symptoms, excl. risk of bias ≥3 | 6 | 0.061 | −0.139, 0.260 | 0.60 | .550 | 0.97 | 0.00 | 0–75 |
| All symptoms, excl. risk of bias ≥2 | 4 | 0.116 | −0.119, 0.352 | 0.97 | .333 | 0.09 | 0.00 | 0–85 |
| Positive symptoms, all eligible studies | 5 | −0.084 | −0.315, 0.147 | −0.71 | .475 | 1.09 | 0.00 | 0–79 |
| Positive symptoms, risk of bias ≥3 | 4 | −0.078 | −0.323, 0.166 | −0.63 | .530 | 1.06 | 0.00 | 0–85 |
| Negative symptoms, all eligible studies | 7 | 0.001 | −0.207, 0.209 | 0.03 | .995 | 4.22 | 0.00 | 0–71 |
| Negative symptoms, excl. risk of bias ≥3 | 5 | 0.006 | −0.223, 0.235 | 0.051 | .958 | 2.28 | 0.00 | 0–79 |
| Social competency outcomes, all eligible studies | 4 | 0.096 | −0.186, 0.379 | 0.67 | .503 | 0.62 | 0.00 | 0–85 |
| SST vs any comparator; longest follow-up pooled | ||||||||
| All symptoms, all eligible studies | 11 | 0.209* | 0.043, 0.375 | 2.46 | .014 | 7.65 | 0.00 | 0–60 |
| All symptoms, excl. risk of bias ≥4 | 9 | 0.252* | 0.075, 0.428 | 2.79 | .005 | 5.17 | 0.00 | 0–65 |
| All symptoms, excl. risk of bias ≥3 | 8 | 0.237* | 0.056, 0.417 | 2.57 | .010 | 4.54 | 0.00 | 0–68 |
| All symptoms, excl. risk of bias ≥2 | 5 | 0.348 | 0.122, 0.574 | 3.02 | .003 | 1.89 | 0.00 | 0–79 |
| Positive symptoms, all eligible studies | 8 | 0.130 | −0.152, 0.412 | 0.91 | .366 | 16.34* | 57.15 | 6–80 |
| Positive symptoms, risk of bias ≥3 | 7 | 0.156 | −0.150, 0.462 | 0.10 | .318 | 15.66* | 61.67 | 13–83 |
| Positive symptoms, risk of bias ≥2 | 4 | 0.282 | −0.196, 0.760 | 1.16 | .247 | 11.57* | 74.08 | 28–91 |
| Negative symptoms, all eligible studies | 10 | 0.228* | 0.025, 0.430 | 2.20 | .028 | 12.51 | 28.70 | 0–65 |
| Negative symptoms, excl. risk of bias ≥3 | 8 | 0.267* | 0.055, 0.478 | 2.47 | .013 | 9.42 | 25.70 | 0–66 |
| Negative symptoms, excl. risk of bias ≥2 | 5 | 0.394* | 0.148, 0.640 | 3.15 | .002 | 4.66 | 14.19 | 0–82 |
| Social competency outcomes, all eligible studies | 8 | −0.100 | −0.964, 0.765 | −0.23 | .821 | 112.22* | 93.76 | 90–96 |
| Social competency outcomes, excl. bias ≥4 | 6 | −0.221 | −1.356, 0.914 | −0.38 | .703 | 111.59* | 95.52 | 93–97 |
| Social competency outcomes, excl. bias ≥3 | 5 | −0.331 | −1.641, 0.978 | 0.50 | .620 | 111.08* | 96.40 | 94–98 |
| Social competency outcomes, excl. bias ≥2 | 4 | −0.425 | −2.165, 1.314 | 0.48 | .632 | 111.08* | 97.30 | 95–98 |
| SST vs any comparator; all follow-up combined | ||||||||
| All symptoms, all eligible studies | 11 | 0.141** | −0.013, 0.294 | 1.79 | .073 | 3.66 | 0.00 | 0–60 |
| All symptoms, excl. risk of bias ≥4 | 10 | 0.169* | 0.007, 0331 | 2.05 | .041 | 1.93 | 0.00 | 0–62 |
| All symptoms, excl. risk of bias ≥3 | 9 | 0.161** | −0.004, 0.326 | 1.91 | .057 | 1.71 | 0.00 | 0–65 |
| All symptoms, excl. risk of bias ≥2 | 6 | 0.231* | 0.033, 0.429 | 2.29 | .022 | 0.09 | 0.00 | 0–75 |
| Positive symptoms, all eligible studies | 8 | 0.045 | −0.158, 0.247 | 0.43 | .664 | 8.51 | 17.76 | 0–61 |
| Positive symptoms, risk of bias ≥4 | 7 | 0.058 | −0.165, 0.280 | 0.51 | .612 | 8.24 | 27.17 | 0–68 |
| Positive symptoms, risk of bias ≥2 | 4 | 0.130 | −0.225, 0.484 | 0.72 | .474 | 6.31 | 52.47 | 0–84 |
| Negative symptoms, all eligible studies | 10 | 0.175** | −0.025, 0.374 | 1.72 | .086 | 12.02 | 25.12 | 0–64 |
| Negative symptoms, excl. risk of bias ≥4 | 8 | 0.204** | −0.009, 0.416 | 1.88 | .060 | 9.40 | 25.55 | 0–66 |
| Negative symptoms, excl. risk of bias ≥2 | 5 | 0.310* | 0.052, 0.567 | 2.36 | .018 | 5.02 | 20.35 | 0–66 |
| Social competency outcomes, all eligible studies | 7 | −0.224 | −1.127, 0.679 | 0.47 | .627 | 91.63* | 93.45 | 89–96 |
| Social competency outcomes, excl. bias ≥4 | 5 | −0.418 | −1.659, 0.823 | −0.66 | .509 | 89.64* | 95.54 | 92–97 |
| Social competency outcomes, excl. bias ≥2 | 4 | −0.608 | −2.107, 0.891 | −0.80 | .427 | 87.94* | 96.59 | 94–98 |
Note: All comparisons were using random model. Risk of bias and subgroup analyses were only included in instances where at least four studies were available for that comparison. *P < .05. **P < .1. PANSS, Positive and Negative Syndromes Scale; CI, confidence Interval; SC, supportive counseling; UCLA-FAST, University of California Los Angeles-Functional Adaptive Skills Training.
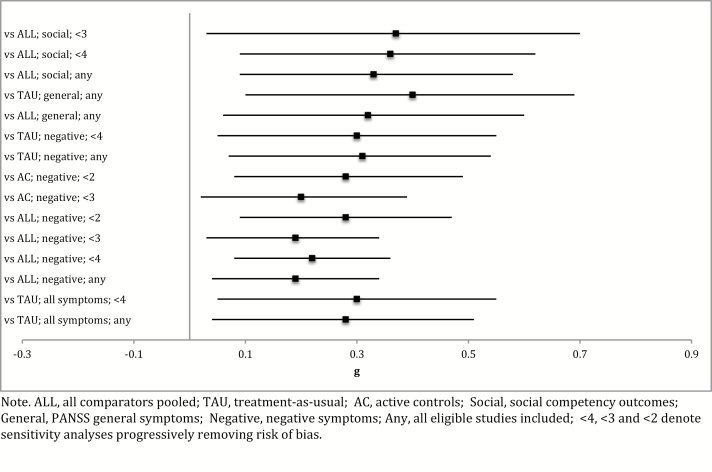
Fig. 2.
Summary forest plot of significant main results in Hedge’s g. ALL, all comparators pooled; TAU, treatment-as-usual; AC, active controls; Social, social competency outcomes; General, PANSS general symptoms; Negative, negative symptoms; Any, all eligible studies included; <4, <3, and <2 denote sensitivity analyses progressively removing risk of bias.
SST was more efficacious for negative symptoms when compared to all comparators pooled, active controls, and TAU. SST was more efficacious compared to pooled comparators (g = 0.19, P = .01) when all eligible studies were included in the analysis. When progressive removal of bias risk was implemented, the effect size gradually increased to g = 0.28 (P = .01). A similar trend was observed for comparison to active controls, where initial comparisons including all studies approached significance while gradual removal of bias resulted in an effect size of g = 0.28 (P = .01). In comparison to TAU, SST was more efficacious when all studies were included (g = 0.31, P = .01), although studies only allowed for removal of studies with a bias risk score of ≥4 (g = 0.30, P = .02). The ≥4 bias comparison was underpowered. SST did not demonstrate superiority against SC for negative symptoms, but this comparison was underpowered with only four studies available. There was no evidence of heterogeneity among negative symptom comparisons.
For Positive and Negative Syndrome Scale (PANSS) general symptoms, SST demonstrated superiority against comparators pooled (g = 0.32, P = .02) and TAU (g = 0.40, P = .01). The limited number of available studies in this symptom domain meant that sensitivity analyses for risk of bias were not possible while comparisons were underpowered. There was no evidence of significant heterogeneity.
Effect of SST for Social Performance
The results for social performance outcome measures are displayed in Table 3. SST was more efficacious when compared to all comparators pooled. This effect size gradually increased from g = 0.33(P = .01) when all eligible studies were included to g = 0.37 (P < .03) when studies scoring ≥3 on bias risk were excluded. The treatment effect was no longer significant on the final sensitivity analysis for studies scoring ≥2 on bias risk, although this comparison was underpowered with only five studies available. SST did not demonstrate significant superiority against active controls or TAU, although the TAU comparison was particularly underpowered. The majority of comparisons in the social performance domain displayed moderate-to-high heterogeneity including significant effects.
Comparison of SST Subtypes
Table 3 provides results of the comparison of the a priori specified SST subtypes. The majority of SST subtype comparisons were underpowered due to limited study availability. In order to assess trends in the data, effects that approached significance (P < .1) were noted and the magnitude of nonsignificant effects was considered. The only subtype that demonstrated significant superiority was SCST, which demonstrated a relatively robust effect size at ≥3 (g = 0.39, P = .01) and ≥2 (g = 0.41, P = .01) bias levels against any comparator pooled for all symptom measures pooled. Generic SST demonstrated an effect size that approached significance for all symptoms pooled (g = 0.36, P = .057), whereas for negative symptoms, a similar magnitude was observed despite the comparison being underpowered (g = 0.27, P < .20). UCLA-FAST approaches showed a nonsignificant trend of inferiority for all symptoms pooled vs any comparator, whereas CBSST comparisons were hampered by limited study availability. Comparisons of CBSST showed no evidence of heterogeneity, whereas generic SST and SCST symptom comparisons did not show significant heterogeneity. Heterogeneity was present for UCLA-FAST comparisons, although decreased as bias risk was reduced. Moderate-to-high heterogeneity was observed across social performance comparisons.
Follow-up
Limited RCT availability meant that meta-analyses on follow-up data were restricted to comparisons at 6-month follow-up, the longest available follow-up, and a pooled follow-up comparison in which an average effect size was calculated across follow-up measurements within each study. This section was restricted to all comparators pooled rather than allowing TAU or active control comparisons. Comparisons were underpowered overall, whereas heterogeneity was consistently low.
SST did not show superiority over any comparator at 6-month follow-up. At the longest available follow-up, SST demonstrated superiority for all symptoms when all eligible RCTs were included (g = 0.20, P = .01), at ≥4 (g = 0.25, P = .01) and at ≥3 (g = 0.24, P = .01) risk of bias levels although lost significance at the most stringent ≥2 risk of bias sensitivity analysis. For negative symptoms, SST demonstrated superiority in all comparisons at the longest available follow-up including when all eligible studies were included (g = 0.23, P = .01) and in the sensitivity analyses for ≥3 (g = 0.27, P = .01) and ≥2 (g = 0.40, P = .002) risk of bias levels. When all follow-up measurements were combined, SST demonstrated superiority for all symptoms at the ≥4 (g = 0.17, P = .04) risk of bias level and also the most stringent ≥2 level (g = 0.23, P = .02). For negative symptoms, the effect of SST approached significance when all eligible studies were included and at the ≥4 risk of bias level. SST demonstrated superiority for negative symptoms at the most stringent sensitivity analysis for the ≥2 risk of bias level (g = 0.31, P = .02). This comparison was underpowered. Follow-up comparison was not available for PANSS general symptoms due to limited study availability.
Publication Bias
Examination of funnel plots and consideration of the trim and fill procedure for effects that demonstrated statistical significance indicated the presence of publication bias in only one comparison. The funnel plot for SST vs all comparators pooled for general symptoms suggested that one study with negative findings had not been published. The trim and fill procedure trimmed one study causing a marginal reduction in the magnitude of effect size in this comparison from g = 0.32 (P ≤ .05) to g = 0.26 (95% CI 0.01, 0.53). The classic fail-safe N procedure suggested that it would require seven missing studies to bring significance below the 0.05 alpha level, whereas Egger’s31 test of the intercept did not demonstrate significance.
Discussion
The current meta-analysis provided a systematic and comprehensive overview of the efficacy of SST for psychosis while also investigating SST subtypes. SST demonstrated superiority for negative symptoms against all comparators pooled, TAU, and active controls with small but reliable differences. SST did not demonstrate superiority over SC for negative symptoms, although this comparison was very low in power. SST also demonstrated superiority against any comparator and TAU for PANSS general symptoms with small-to-medium effects. SST was superior to TAU when pooling all symptom measures but did not demonstrate superiority against comparators pooled, active control, or SC. There were no significant effects on positive symptoms. SST demonstrated superiority only against comparators pooled for social competency measures, although this effect lost significance as bias risk and power decreased. Significant effects for social outcomes were overall marginally larger than those for negative symptoms, although significant heterogeneity was present across significant findings in this category while effects were not maintained against active controls. In SST subtype comparisons, only SCST demonstrated superiority to pooled comparators. Effects of SST vs all comparators for all symptoms pooled and negative symptoms were maintained on a proportion of follow-up comparisons.
As hypothesized, SST demonstrated superiority for negative symptoms including in comparison against active controls, which is the most stringent comparison category. SST also demonstrated beneficial effects on those comparisons possible for general symptoms. The overall trend in analyses for both negative and PANSS general symptoms showed that the magnitude of SST effect increased as risk of bias decreased, suggesting these effects may be robust. There was, however, still a minimal level of risk of bias present in the RCTs pooled to provide these conclusions because no RCT achieved the lowest possible risk of bias score. Sensitivity analyses for social outcomes did not follow this trend, with the effect size decreasing and findings losing significant when bias was minimized. Similarly, many comparisons allowed only the least stringent category of sensitivity analysis due to limited availability of methodologically strong RCTs. Comparisons in the social performance domain displayed moderate-to-high heterogeneity. This heterogeneity may be a result of combining a high number of outcome measures that were not designed to measure a narrowly defined construct. Our combination of these measures may therefore indicate that a number of related but distinct outcomes were included, whereas a lack of robust significant effects in this domain may also be related to the heterogeneity in the included outcomes.
Although SCST demonstrated superiority to pooled comparators again with magnitude increasing as bias decreased, no other SST subtypes demonstrated superiority in the context of low power across comparisons. There were two effects approaching significance: generic SST at ≥4 risk of bias sensitivity analysis and SCST at ≥3 risk of bias sensitivity analysis, whereas UCLA-FAST performed poorly, despite having the highest statistical power. This may therefore suggest that “practical” life skills approaches have less beneficial impact upon symptoms than other subtypes. It is difficult to draw any conclusion regarding CBSST due to limited study availability. The identification of SST subtypes in meta-analysis may therefore become more relevant as the literature develops and future meta-analyses may benefit from increased study availability to bolster categories. Further research in this area, which can comprehensively compare the effectiveness of SST subtypes, may help influence the development of effective SST interventions.
The beneficial effects of SST were not evident at 6-month follow-up, which was underpowered. Robust effects were however maintained for negative symptoms at the longest available follow-up point and in a less robust manner for all symptoms, whereas combining follow-up points also demonstrated similar lasting effects of SST. SST has faced criticism that learning does not generalize well to real-life situations.16 These findings indicate that SST has potential as an intervention that maintains effects outside the therapy group, although demonstrating generalizability and longevity remain important.
The effect sizes reported for SST for negative symptoms (g = 0.2–0.03) are marginally greater than those reported for CBT for positive symptoms and marginally smaller than those reported for antipsychotics,8,9 whereas current evidence does not support CBT for negative symptoms.14If we consider CBT as an intervention addressing positive symptoms and SST for negative symptoms, each intervention has effects of roughly equivalent magnitude for its target area.36 As discussed, SST is not recommended as a stand-alone intervention by NICE and therefore is not routinely implemented in the NHS.10 Furthermore, no UK RCTs met inclusion criteria for this meta-analysis, whereas many meet criteria for CBT meta-analyses.9,15,37,38 It is possible that a culture toward cognitive-behavioral, formulation-based interventions is limiting the consideration of alternative approaches that demonstrate similar efficacy. The group-based style of SST may lend itself well to application within a CMHT environment and has the potential to act as a cost-effective means of addressing negative symptoms, whereas improved care matching protocols may develop to help identify which patients may benefit most from the range of available interventions and depending on their capacity to engage.39
The positive findings for SST on general psychopathology are also of interest. The PANSS general psychopathology subscale may be conceptualized as a measure of general distress including depression and anxiety, which have been identified as factorial dimensions within psychosis symptomatology.40 Understanding of depression as an integral part of psychosis is limited as are targeted interventions. The small-to-medium effect sizes shown for SST in this domain suggest that targeting general psychopathology is worthy of consideration for the broader recovery agenda41 while contemporary research challenges the traditionally prevalent assumption that psychosis and depression are aetiologically distinct.40 Considered broadly, these findings suggest the importance of developing interventions for psychosis populations that carefully consider the symptom and functioning domains measured by negative and general symptom scales.
It should also be recognized that negative symptoms represent heterogeneous sequelae within psychosis. Recent research supports a two-factor structure within negative symptoms in which expressive or neurocognitive deficits are associated primarily with limited life functioning, whereas a second factor representing limited social motivation is associated with depressive symptomatology.41,42 Research on intervention targeting specific subgroups within negative symptoms is in its infancy.43 Although simultaneously considering our findings on general symptoms, the potential crossover between negative symptoms and depressive symptomatology has implications for the development of effective interventions.
On a macro level, this review also provides support that small but reliable differences exist between psychological interventions, particularly on the outcomes targeted specifically by the intervention. This contradicts the Dodo verdict that all psychological interventions are equivalent because SST retained superiority for negative symptoms observed elsewhere.9,44 Small effect sizes and a number of nonsignificant comparisons vs active controls may also be interpreted as supportive of the premise that interventions are roughly equivalent although the difficulty of low power in these comparisons should not be dismissed. Wampold45,46 highlights the tendency of meta-analyses of psychological interventions to establish targeted, symptom-specific improvement as opposed to improved general functioning. The observed effect on PANSS general symptoms suggests that improvement may occur on outcomes capturing comorbidity, although our methodology does not have the sophistication to specify the mechanism of such improvements.
There were a number of limitations including those inherent to meta-analyses and those specific to this review. With regard to the literature, although 27 RCTs were included, participant numbers in many trials were low.47 Many comparisons were therefore hampered by low power and there were not enough high quality studies minimizing bias risk to allow comparison at the lowest risk of bias level. This meant that any significant finding is still susceptible to some degree of potential bias.
Based on our comparison strategy, another limitation was that many RCTs had to be excluded due to the mixed nature of interventions; eg, integrating medication, exercise, or other psychological therapies alongside SST. It was beyond the scope of this review to consider these interventions, although a narrative systematic review may help provide clarity on this burgeoning literature. Similarly, although we attempted to address the issue via joint decision-making, our categorization of SST subtypes retains a degree of subjectivity while subtypes may contain heterogeneity. Nevertheless, the first meta-analytic consideration of SST subtypes provides guidance for future reviewers as this literature develops.
The lack of translation capability should also be considered a limitation in this review because we were unable to fully assess ten potential articles for inclusion. A final limitation is that a wider range of outcomes are relevant to recovery from psychosis than those included in this review, eg, quality of life, neurocognitive function, relapse, or employment. Considering all such outcomes was beyond the scope of our project; therefore, depending on study availability, future research may consider them.
A further limitation of this review is that neither the methodology nor the scope of this review allow insight into the mechanism of change by which SST modifies symptoms or social performance. In light of our findings, it is possible that SST exerts secondary change in negative symptoms via the primary target of social functioning, whereas an alternative possibility is that SST has direct impact upon negative symptoms such as poor rapport, emotional withdrawal, and passivity which in turn improves social performance. Moreover, there is considerable conceptual crossover among negative symptoms, general symptoms, and social functioning. Future research dismantling the mechanisms of SST is therefore warranted.
Taken in the context of wider research findings, the magnitude of effects demonstrated by SST for negative and general symptoms is relatively comparable to other interventions including the extent of benefit shown by antipsychotic and antidepressant medication.8 As aforementioned, we recognize that because the majority of participants in included RCTs would have been maintained on medication, the beneficial effects of SST are over and above any existing pharmacological effect on symptoms, whereas the efficacy of SST for unmedicated participants remains unknown.
The results of this meta-analysis suggest that SST has the potential for wider clinical application, whereas the level of evidence demonstrated for SST contradicts its exclusion by NICE in the UK.10 The effect sizes reported are impressive for a group-based psychological intervention suggesting that SST may have potential as a cost-effective alternative to individual therapies addressing negative and general symptoms in healthcare systems struggling to provide routine psychological intervention while SST may also provide a beneficial adjunct to CBTp focused on appraisal and positive symptoms.
Further high-quality outcome research may help clarify doubts regarding the applicability and durability of SST in practice. At the very least, an RCT with stringent methodology applying SST for negative symptoms in a routine mental healthcare setting is warranted. Any future research may also benefit from integrating a cost-effectiveness analysis. Future SST research must focus upon further reducing risk of bias among RCTs and therefore allowing equivalence to CBT methodology alongside further addressing the concerns regarding generalizability and longevity. It is therefore important that methodologically stringent RCTs continue to integrate follow-up assessments on primary outcome measures, whereas the integration of booster sessions or any similar attempt to prolong beneficial effects, trouble-shoot, and increase applicability to real-life settings may help address pre-existing concerns. The significant findings on a proportion of follow-up comparisons suggest that further development of SST is warranted.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Conflict of Interest Statement
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Supplementary Material
References
- 1. Wallace CJ, Nelson CJ, Liberman RP et al. . A review and critique of social skills training with schizophrenic patients. Schizophr Bull. 1980;6:42–63. [DOI] [PubMed] [Google Scholar]
- 2. Gohar SM, Hamdi E, El Ray LA, Horan WP, Green MF. Adapting and evaluating a social cognitive remediation program for schizophrenia in Arabic. Schizophr Res. 2013;148:12–17. [DOI] [PubMed] [Google Scholar]
- 3. Horan WP, Kern RS, Shokat-Fadai K, Sergi MJ, Wynn JK, Green MF. Social cognitive skills training in schizophrenia: an initial efficacy study of stabilized outpatients. Schizophr Res. 2009;107:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Granholm E, Holden J, Link PC, McQuaid JR. Randomized clinical trial of cognitive behavioral social skills training for schizophrenia: improvement in functioning and experiential negative symptoms. J Consult Clin Psychol. 2014;82:1173–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patterson TL, Mausbach BT, McKibbin C, Goldman S, Bucardo J, Jeste DV. Functional adaptation skills training (FAST): a randomized trial of a psychosocial intervention for middle-aged and older patients with chronic psychotic disorders. Schizophr Res. 2006;86:291–299. [DOI] [PubMed] [Google Scholar]
- 6. Xiang YT, Weng YZ, Li WY et al. . Efficacy of the Community Re-Entry Module for patients with schizophrenia in Beijing, China: outcome at 2-year follow-up. Br J Psychiatry. 2007;190:49–56. [DOI] [PubMed] [Google Scholar]
- 7. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 8. Fusar-Poli P, Papanastasiou E, Stahl D et al. . Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41:892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turner D, van der Gaag M, Karyotaki E, Cuijpers P. Psychological interventions for psychosis: a meta-analysis of comparative outcome studies. Am J Psychiatry. 2014;171:523–538. [DOI] [PubMed] [Google Scholar]
- 10. National Institute for Clinical Excellence. Psychosis and schizophrenia in adults: prevention and management. NICE guidelines. 2014 [CG178]. [PubMed]
- 11. Lehman AF, Lieberman JA, Dixon LB, et al. . Practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. 2004;161(2 Suppl). [PubMed] [Google Scholar]
- 12. Hagen R, Turkington D, Berge T.. CBT for Psychosis: A Symptom-Based Approach. Routledge; 2010. [Google Scholar]
- 13. Wykes T, Steel C, Everitt B, Tarrier N. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull. 2008;34:523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Velthorst E, Koeter M, van der Gaag M et al. . Adapted cognitive-behavioural therapy required for targeting negative symptoms in schizophrenia: meta-analysis and meta-regression. Psychol Med. 2015;45:453–465. [DOI] [PubMed] [Google Scholar]
- 15. National Institute for Health and Clinical Excellence. Schizophrenia: core interventions in the treatment and management of schizophrenia in adults in primary and secondary care clinical guideline. Natl Inst Heal Care Excell London. 2009;82:1–399. [Google Scholar]
- 16. National Institute for Health and Clinical Excellence. Schizophrenia (update): Full Guideline. London: Natl Ins Heal Care Excell. 2009;1––399.. [Google Scholar]
- 17. Pilling S, Bebbington P, Kuipers E et al. . Psychological treatments in schizophrenia: II. Meta-analyses of randomized controlled trials of social skills training and cognitive remediation. Psychol Med. 2002;32:783–791. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L34823331. [DOI] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9, W64. [DOI] [PubMed] [Google Scholar]
- 19. Almerie MQ utayba, Okba Al Marhi M, Jawoosh M et al. . Social skills programmes for schizophrenia. Cochrane database Syst Rev. 2015;6:CD009006. doi:10.1002/14651858.CD009006.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kurtz MM, Richardson CL. Social cognitive training for schizophrenia: a meta-analytic investigation of controlled research. Schizophr Bull. 2012;38:1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins JP, Altman DG, Gøtzsche PC et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bucci P, Piegari G, Mucci A et al. . Neurocognitive individualized training versus social skills individualized training: a randomized trial in patients with schizophrenia. Schizophr Res. 2013;150:69–75. [DOI] [PubMed] [Google Scholar]
- 23. Lee TY, Chang SC, Chu H et al. . The effects of assertiveness training in patients with schizophrenia: a randomized, single-blind, controlled study. J Adv Nurs. 2013;69:2549–2559. [DOI] [PubMed] [Google Scholar]
- 24. Anzai N, Yoneda S, Kumagai N, Nakamura Y, Ikebuchi E, Liberman RP. Training persons with schizophrenia in illness self-management: a randomized controlled trial in Japan. Psychiatr Serv. 2002;53:545–547. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L34454171. [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, Roberts DL, Xu B, Cao R, Yan M, Jiang Q. Social cognition and interaction training for patients with stable schizophrenia in Chinese community settings. Psychiatry Res. 2013;210:751–755. [DOI] [PubMed] [Google Scholar]
- 26. Velligan DI, Roberts DL, Maples-Aguilar NJ, Medellin E. A randomized pilot study of motivation enhancement therapy (move). Schizophr Bull. 2015;41:S338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schwarzer G, Schwarzer MG. Package meta. Meta-analysis with R. 2016:1–2. [Google Scholar]
- 28. Viechtbauer W. Metafor: Meta-analysis package for R. R Packag version 2010;2010:0–1. [Google Scholar]
- 29. Deeks JJ, Higgins J, Altman DG. Analysing data and undertaking meta-analyses. Cochrane Handb Syst Rev Interv Cochrane B Ser. 2008:243–296. [Google Scholar]
- 30. Borenstein M, Hedges LV, Higgins J, Rothstein HR.. Introduction to meta-analysis. Chichester, United Kingdom: John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 31. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sterne JA, Egger M, Moher D.. Addressing reporting biases in Cochrane handbook for systematic reviews of interventions. Cochrane Book Series. In: Higgins JP, Green S eds. The Cochrane Collaboration. London, United Kingdom; 2008. [Google Scholar]
- 33. Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 34. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cuijpers P, Ph D.. Meta-Analyses in Mental Health Research. A Practical Guide. Amsterdam: Vrije Universiteit; 2016. [Google Scholar]
- 36. van der Gaag M, Valmaggia LR, Smit F. The effects of individually tailored formulation-based cognitive behavioural therapy in auditory hallucinations and delusions: a meta-analysis. Schizophr Res. 2014;156:30–37. [DOI] [PubMed] [Google Scholar]
- 37. Lynch D, Laws KR, McKenna PJ. Cognitive behavioural therapy for major psychiatric disorder: does it really work? A meta-analytical review of well-controlled trials. Psychol Med. 2010;40:9–24. [DOI] [PubMed] [Google Scholar]
- 38. Jauhar S, McKenna PJ, Radua J, Fung E, Salvador R, Laws KR. Cognitive-behavioural therapy for the symptoms of schizophrenia: systematic review and meta-analysis with examination of potential bias. Br J Psychiatry. 2014;204:20–29. [DOI] [PubMed] [Google Scholar]
- 39. Turner DT, MacBeth A, Larkin A, Livingstone K, Campbell A, Hutton P. A randomised experimental manipulation of the jumping-to-conclusions bias in psychosis; impact of brief meta-cognitive training on capacity. 2017.
- 40. Upthegrove R, Marwaha S, Birchwood M. Depression and schizophrenia: cause, consequence or trans-diagnostic issue?Schizophr Bull. 2016;43:sbw097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liemburg E, Castelein S, Stewart R, van der Gaag M, Aleman A, Knegtering H; Genetic Risk and Outcome of Psychosis (GROUP) Investigators Two subdomains of negative symptoms in psychotic disorders: established and confirmed in two large cohorts. J Psychiatr Res. 2013;47:718–725. [DOI] [PubMed] [Google Scholar]
- 42. Stiekema AP, Liemburg EJ, van der Meer L et al. . Confirmatory factor analysis and differential relationships of the two subdomains of negative symptoms in chronically ill psychotic patients. PLoS One. 2016;11:e0149785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Staring AB, Ter Huurne MA, van der Gaag M. Cognitive behavioral therapy for negative symptoms (CBT-n) in psychotic disorders: a pilot study. J Behav Ther Exp Psychiatry. 2013;44:300–306. [DOI] [PubMed] [Google Scholar]
- 44. Schizophrenia Commission. The abandoned illness: a report from the Schizophrenia Commission. London: Rethink Mental Illness; 2012. [Google Scholar]
- 45. Wampold BE, Imel ZE.. The Great Psychotherapy Debate: The Evidence for What Makes Psychotherapy Work. Abingdon, United Kingdom: Routledge; 2015. [Google Scholar]
- 46. Wampold BE, Flückiger C, Del Re AC et al. . In pursuit of truth: a critical examination of meta-analyses of cognitive behavior therapy. Psychother Res. 2017;27:14–32. [DOI] [PubMed] [Google Scholar]
- 47. Gil Sanz D, Diego Lorenzo M, Bengochea Seco R et al. . Efficacy of a social cognition training program for schizophrenic patients: a pilot study. Span J Psychol. 2009;12:184–191. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L354747274. [DOI] [PubMed] [Google Scholar]
- 48. Bellack AS, Hersen M, Himmelhoch JM. A comparison of social-skill training, pharmacotherapy and psychotherapy for depression. Behav Res Ther. 1983;21:101–107. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/473/CN-00187473/frame.html. [DOI] [PubMed] [Google Scholar]
- 49. Tas C, Danaci AE, Cubukcuoglu Z, Brüne M. Impact of family involvement on social cognition training in clinically stable outpatients with schizophrenia—a randomized pilot study. Psychiatry Res. 2012;195:32–38. [DOI] [PubMed] [Google Scholar]
- 50. Roberts DL, Combs DR, Willoughby M et al. . A randomized, controlled trial of Social Cognition and Interaction Training (SCIT) for outpatients with schizophrenia spectrum disorders. Br J Clin Psychol. 2014;53:281–298. [DOI] [PubMed] [Google Scholar]
- 51. Bowie CR, McGurk SR, Mausbach B, Patterson TL, Harvey PD. Combined cognitive remediation and functional skills training for schizophrenia: effects on cognition, functional competence, and real-world behavior. Am J Psychiatry. 2012;169:710–718. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L365263519. [DOI] [PubMed] [Google Scholar]
- 52. Chien H, Ku C, Lu R, Chu H, Tao Y, Chou K. Effects of Social Skills Training on Improving Social Skills of Patients With Schizophrenia. 2003;XVII:228–236. [DOI] [PubMed] [Google Scholar]
- 53. Choi KH, Kwon JH. Social cognition enhancement training for schizophrenia: a preliminary randomized controlled trial. Community Ment Health J. 2006;42:177–187. [DOI] [PubMed] [Google Scholar]
- 54. Dobson DJ, McDougall G, Busheikin J, Aldous J. Effects of social skills training and social milieu treatment on symptoms of schizophrenia. Psychiatr Serv. 1995;46:376–380. [DOI] [PubMed] [Google Scholar]
- 55. Granholm E, McQuaid JR, McClure FS et al. . A randomized, controlled trial of cognitive behavioral social skills training for middle-aged and older outpatients with chronic schizophrenia. Am J Psychiatry. 2005;162:520–529. [DOI] [PubMed] [Google Scholar]
- 56. Granholm E, McQuaid JR, McClure FS et al. . Randomized controlled trial of cognitive behavioral social skills training for older people with schizophrenia: 12-month follow-up. J Clin Psychiatry. 2007;68:730–737. http://www.ncbi.nlm.nih.gov/pubmed/17503982. [DOI] [PubMed] [Google Scholar]
- 57. Granholm E, Holden J, Link PC, McQuaid JR, Jeste DV. Randomized controlled trial of cognitive behavioral social skills training for older consumers with schizophrenia: defeatist performance attitudes and functional outcome. Am J Geriatr Psychiatry. 2013;21:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hayes RL, Halford WK, Varghese FT. Social skills training with chronic schizophrenic patients: Effects on negative symptoms and community functioning. Behav Ther. 1995;26:433–449. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L25274891. [Google Scholar]
- 59. Hogarty GE, Anderson CM. Medication, family psychoeducation, and social skills training: first year relapse results of a controlled study. Psychopharmacol Bull. 1986;22:860–862. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L17677696. [PubMed] [Google Scholar]
- 60. Hogarty GE, Anderson CM, Reiss DJ, Kornblith SJ, Greenwald DP, Ulrich RF. Family psychoeducation, social skills training, and maintenance chemotherapy in the aftercare treatment of schizophrenia. II. Two-year effects of a controlled study on relapse and adjustment. Arch Gen Psychiatry. 1991;48:340–347. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/457/CN-00660457/frame.html. [DOI] [PubMed] [Google Scholar]
- 61. Horan WP, Kern RS, Tripp C et al. . Efficacy and specificity of Social Cognitive Skills Training for outpatients with psychotic disorders. J Psychiatr Res. 1113;45:1113–1122. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=emed10&AN=2011402493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lecomte T, Leclerc C, Wykes T, Wallace CJ, Spidel A. Group cognitive behaviour therapy or social skills training for individuals with a first episode of psychosis? Results of a randomized controlled trial [NCT00358709]. J Nerv Ment Dis. 2008;196:866–875. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/338/CN-00783338/frame.html. [DOI] [PubMed] [Google Scholar]
- 63. Lecomte T, Leclerc C, Wykes T, Spidel A. Group CBT for early psychosis—Are there still benefits 1 year later?Early Interv Psychiatry. 2010;4:150 http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L70588548. [Google Scholar]
- 64. Liberman RP, Mueser KT, Wallace CJ. Social skills training for schizophrenic individuals at risk for relapse. Am J Psychiatry. 1986;143:523–526. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/968/CN-00214968/frame.html. [DOI] [PubMed] [Google Scholar]
- 65. Wallace CJ, Liberman RP. Social skills training for patients with schizophrenia: a controlled clinical trial. Psychiatry Res. 1985;15:239–247. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/455/CN-00039455/frame.html. [DOI] [PubMed] [Google Scholar]
- 66. Lukoff D, Wallace CJ, Liberman RP, Burke K. A holistic program for chronic schizophrenic patients. Schizophr Bull. 1986;12:274–282. [DOI] [PubMed] [Google Scholar]
- 67. Liberman RP, Wallace CJ, Blackwell G, Kopelowicz A, Vaccaro JV, Mintz J. Skills training versus psychosocial occupational therapy for persons with persistent schizophrenia. Am J Psychiatry. 1998;155:1087–1091. http://www.ncbi.nlm.nih.gov/pubmed/9699698. [DOI] [PubMed] [Google Scholar]
- 68. Marder SR, Wirshing WC, Mintz J et al. . Two-year outcome of social skills training and group psychotherapy for outpatients with schizophrenia. Am J Psychiatry. 1996;153:1585–1592. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L26406169. [DOI] [PubMed] [Google Scholar]
- 69. Ng RMK, Cheung MSL. Social skills training in Hong Kong Chinese patients with chronic schizophrenia. Hong Kong J Psychiatry. 2007;16:14–20. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L46648940. [Google Scholar]
- 70. Patterson TL, Bucardo J, McKibbin CL et al. . Development and pilot testing of a new psychosocial intervention for older Latinos with chronic psychosis. Schizophr Bull. 2005;31:922–930. [DOI] [PubMed] [Google Scholar]
- 71. Rus-Calafell M, Gutiérrez-Maldonado J, Ortega-Bravo M, Ribas-Sabaté J, Caqueo-Urízar A. A brief cognitive-behavioural social skills training for stabilised outpatients with schizophrenia: a preliminary study. Schizophr Res. 2013;143:327–336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.