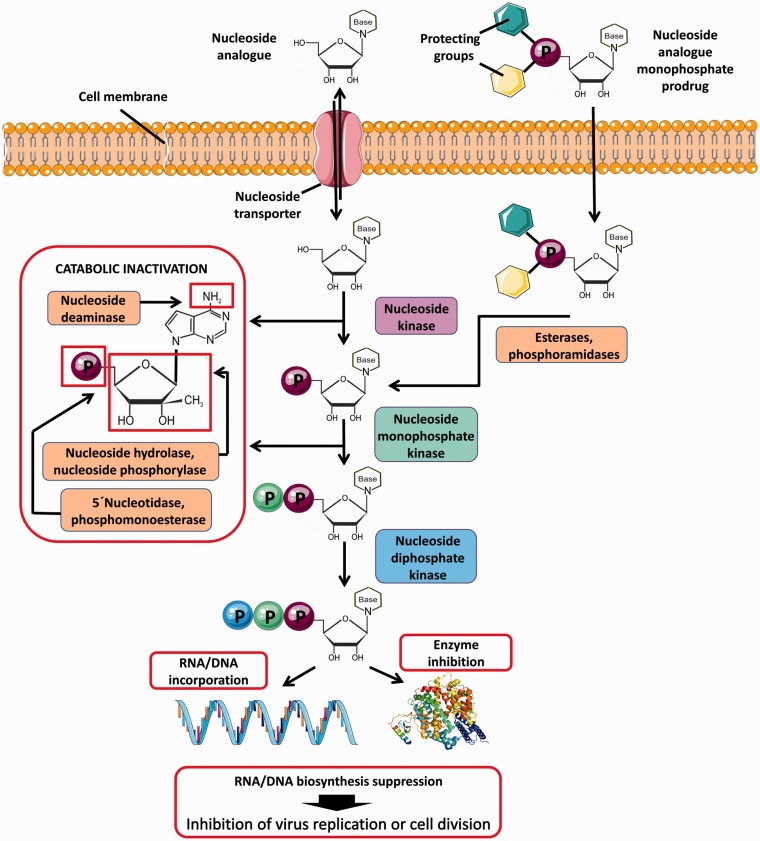
Figure 1.
Intracellular uptake and metabolism of nucleoside analogs and nucleoside analog prodrugs. Nucleoside analogs enter cells through specific plasma membrane nucleoside transporters. Inside the cell, the compounds are phosphorylated by cellular nucleoside kinases resulting in formation of nucleoside mono-, di-, and triphosphates. The first kinase phosphorylation is the rate-limiting step of the triphosphate conversion, which can be overcome by the monophosphate prodrug approach based on the introduction of a phosphorylated group into the 5′ nucleoside position. The phosphorylated group includes protecting moieties to increase hydrophobicity and facilitate the cellular uptake of the prodrug. Monophosphate prodrugs enter cells independently of membrane transporters and the protecting groups are removed by intracellular esterases or phosphoramidases after cell penetration. The triphosphates of nucleoside species represent the active forms of nucleoside analogs that act by inhibiting cellular or viral enzymes, such as DNA/RNA polymerases. During DNA/RNA replication, nucleoside analogs are incorporated into nascent DNA or RNA chains resulting in termination of nucleic acid synthesis or in accumulation of mutations in viral genomes to suppress viral replication due to error catastrophe. At normal physiological conditions, intracellular nucleoside concentrations are maintained at low levels due to nucleoside/nucleotide catabolic pathways, such as deamination (oxidation) of heterocyclic base, hydrolysis or phosphorolysis of heterocyclic base, and hydrolysis of phosphomonoester bonds. These catabolic reactions also concern most nucleoside analogs containing the natural N-glycosidic bond and/or the degradable functional groups of the heterocyclic base. Figure created using Servier Medical Art available on www.servier.com.