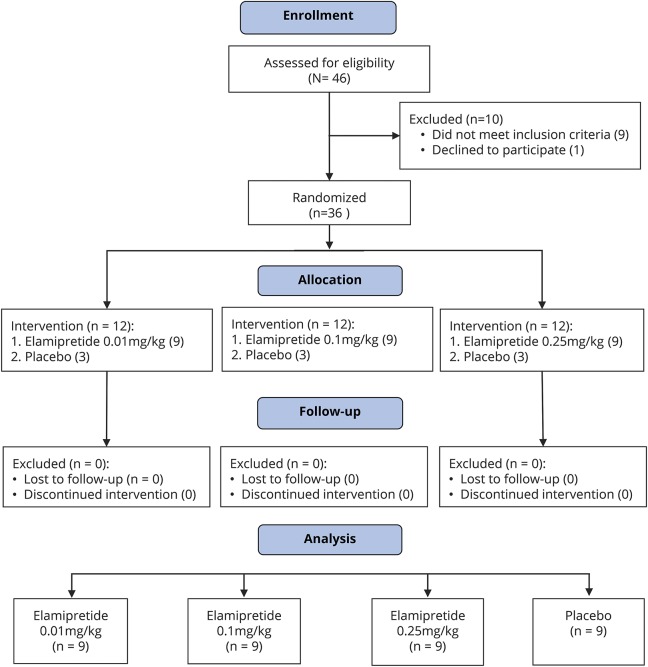
Figure 1. CONSORT 2010 flowchart for the MMPOWER trial.
Forty-six participants were screened for the trial. Ten were excluded because of major health concerns, including cardiac and neurologic disorders (arrhythmias, severe ataxia), inability to perform the 6MWT, morbid obesity, recent changes in medical management, deemed unstable, or unwilling to participate in the trial procedures. Of the 36 participants enrolled, none dropped out or were lost to follow-up. All participants performed the required trial procedures and were included, and their information was used in the final analysis. CONSORT = Consolidated Standards of Reporting Trials; MMPOWER = A Study Investigating the Safety, Tolerability, and Efficacy of MTP-131 for the Treatment of Mitochondrial Myopathy; 6MWT = 6-minute walk test.