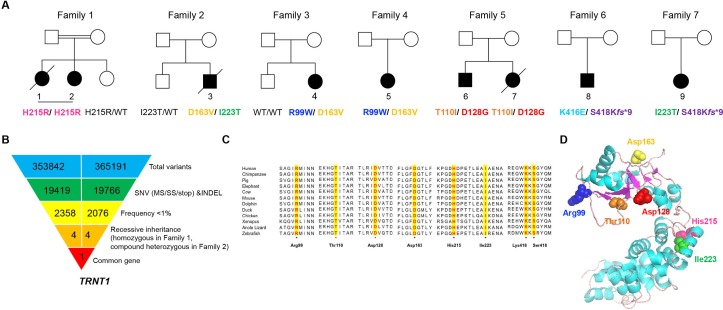
Figure 1.
Biallelic mutations in TRNT1 in the NHGRI cohort of patients. (A) Pedigrees of nine patients with SIFD. (B) Schematic representation of the exome data filtering approach leading to the identification of TRNT1 as the unique common gene in the first two families. (C) Evolutionary conservation of SIFD-associated mutations in this cohort. (D) In silico modelling of TRNT1 mutations based on the crystal structure of human TRNT1 (1ou5) (NP_001289875). Residues Arg99, Asp163, Thr110 and Asp128 are located within the head domain of TRNT1 and close to the catalytic residues Asp77, Asp79 and Glu121. Residues His215 and Ile223 are located in the neck domain. Lys416 and Ser418 are in the tail domain of the enzyme beyond the resolved crystal structure. (The PyMOL Molecular Graphics System, Schrödinger). SIFD, sideroblastic anaemia with immunodeficiency, fevers and developmental delay.