Abstract
Background
Endotracheal tubes provide an abiotic surface on which bacteria and fungi form biofilms, and the release of endotoxins and planktonic organisms can cause damaging inflammation and infections.
Objectives
Ceragenins are small molecule mimics of antimicrobial peptides with broad-spectrum antibacterial and antifungal activity, and a ceragenin may be used to provide antimicrobial protection to the abiotic surface of an endotracheal tube.
Methods
A hydrogel film, containing CSA-131, was generated on endotracheal tubes. Elution of CSA-131 was quantified in drip-flow and static systems, antifungal and antibacterial activity was measured with repeated inoculation in growth media, biofilm formation was observed through electron microscopy, safety was determined by intubation of pigs with coated and uncoated endotracheal tubes.
Results
Optimized coatings containing CSA-131 provided controlled elution of CSA-131, with concentrations released of less than 1 μg/mL. The eluting ceragenin prevented fungal and bacterial colonization of coated endotracheal tubes for extended periods, while uncoated tubes were colonized by bacteria and fungi. Coated tubes were well tolerated in intubated pigs.
Conclusions
Thin films containing CSA-131 provide protection against microbial colonization of endotracheal tubes. This protection prevents fungal and bacterial biofilm formation on the tubes and reduces endotoxin associated with tubes. This coating is well suited for decreasing the adverse effects of intubation associated with infection and inflammation.
Introduction
Tissues in the airways provide innate immune defences against microbial colonization, and key components of these defences are antimicrobial peptides (AMPs).1,2 These defences limit microbial transfer from the highly colonized oropharynx to the nearly sterile lung tissues. Nearly 50 million patients worldwide are intubated annually with endotracheal tubes (ETTs) to assist breathing. The abiotic surfaces of ETTs provide a breeding ground on which microbes can readily form biofilms,3–5 because the surfaces do not provide an innate immune function comparable to surrounding tissues. Biofilms that form on these abiotic surfaces are a nidus for continual inoculation of surrounding tissues while providing an avenue for microbial transfer into the lungs leading to pneumonia. Ventilator-associated pneumonia remains a major healthcare burden,5,6 adding to time in intensive care, costs of hospitalization and increasing the use of antibiotics. Infections associated with mechanical ventilation lead to endotoxin-driven inflammation,7 which in turn can result in sepsis and remote organ damage (e.g. acute kidney injury), brain damage in neonates8,9 and delirium in elderly patients.10,11 Prevention of biofilm growth on ETTs may result in significant clinical improvements, reduced antibiotic usage, emergence of fewer MDR bacteria, reduction of endotoxin-driven adverse clinical outcomes and shorter duration of stay in the ICU.
It is increasingly recognized that microbial colonization of ETTs is polymicrobial.3,4,12 The presence of different microbial pathogens in close proximity to each other in the biofilm matrix enables horizontal gene transfer of plasmids encoding resistance to conventional antibiotics and thereby facilitates the emergence of new drug-resistant strains.13,14 Initial studies of ETT colonization focused on both Gram-positive and Gram-negative pathogens; however, fungi are now understood to contribute to ETT colonization.3,4,12 Of particular concern are highly drug-resistant organisms. For example, while colistin is considered the antibiotic of ‘last resort’ for Gram-negative bacteria, highly colistin-resistant bacteria have been isolated from a variety of tissues.15–17 Additionally, due to the relatively few antifungal agents available, recent identification of Candida auris as a drug-resistant fungal pathogen has led to fears of untreatable infections.18
Efforts to prevent ventilator-associated infections have included use of systemic antibiotics19–21 as well as silver-releasing ETTs.22,23 Recent studies by Stulik et al.19 and Burnham & Kollef20 have shown that systemic administration of antibiotics has little effect on airway colonization, and this ineffectiveness is likely due to the rapidity with which bacteria form biofilms and the insensitivity of these biofilms to most antibiotics. Silver-releasing ETTs showed promise in preventing bacterial colonization,22,23 with a concomitant reduction in risk of infection in patients ventilated for more than 24 h. However, silver-releasing ETTs have not gained widespread use in hospitals.23
An attractive approach for prevention of microbial colonization of ETTs would be to provide an innate immune-like function on their abiotic surfaces. This function would necessarily mimic the activities of endogenous AMPs: broad-spectrum antibacterial and antifungal activity and the ability to sequester bacterial endotoxins to minimize inflammation. The use of AMPs to provide an innate immune-like function to ETTs has been proposed.24 However, there are several challenges in the clinical use of AMPs, including the relatively high cost of producing peptide therapeutics, as compared with their small-molecule counterparts, and the susceptibility of AMPs to degradation by proteases.25
We have developed small-molecule, non-peptide mimics of AMPs, termed ceragenins,26,27 that display broad-spectrum antibacterial and antifungal activity, including activity against drug-resistant organisms. Lead ceragenins are active against highly colistin-resistant Gram-negative bacteria,28,29 and they retain activity against drug-resistant Candida spp.30 As mimics of AMPs, ceragenins also associate with endotoxins,31 inhibiting the processes that lead to release of inflammatory cytokines. As small molecules, ceragenins can be readily prepared at a large scale, and because they are not peptide based, they are not substrates for proteases.
As functional mimics of AMPs, ceragenins are well suited for providing innate immune-like functions to the abiotic surfaces of medical devices. Herein we describe the incorporation of a lead ceragenin, CSA-131 (Figure 1), into a thin film, comprised of a lubricious hydrogel, on ETTs. The hydrogel film provides a reservoir of the ceragenin and facilitates controlled release of the ceragenin over extended periods. As the ceragenin is released, it prevents bacterial and fungal biofilm formation and sequesters endotoxins. In its optimized form, the coating is applied via dip coating with a mixture containing the ceragenin, followed by polymerization of the hydrogel. The resulting coating can be sterilized by ethylene oxide without adduct formation. In vivo experiments showed that the coated ETT is well tolerated, and no systemic exposure to ceragenin was observed. The US FDA has recognized the need for ETTs that prevent microbial colonization, and the coated ETT described herein was recently granted Expedited Access Pathway designation pursuant to the 21st Century Cures Act.
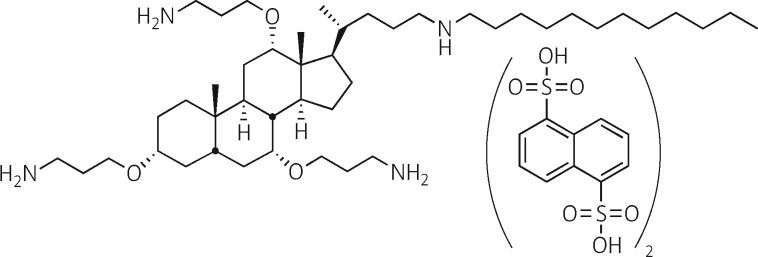
Figure 1.
Structure of CSA-131(NDSA).
Materials and methods
Coating
CSA-131(NDSA) was jet milled (Fluid Energy, Telford, PA) to give particles (< 1 μm) and added to Hydromer Coating Solution 2018–20M (Hydromer, Branchburg, NJ) in quantities equal to 10% (w/w) relative to the amount of non-volatile material in the coating solution. To prepare ETT segments, tubes [7.5 mm internal diameter (id), Flexicare, Irvine, CA] were cut into 5 mm segments. For coating, intact tubes and tube segments were washed with isopropanol and hexanes and fully dried. Tubes and segments were dip coated, generating a uniform coating on the inner and outer lumens, and the coating was then cured at 71.1 °C for 1 h. With tube segments, weight differences were determined and used to calculate average coating thickness. Coated tubes and tube segments were subjected to standard ethylene oxide (ETO) sterilization procedures (Steris, San Diego, CA), and ETO-treated segments were used to quantify ETO adduct formation with CSA-131 via mass spectrometry (Agilent 6230 TOF, Santa Clara, CA). To determine the total amount of CSA-131(NDSA) in the coatings, segments (5 mm) of coated ETTs were immersed (1 mL) in an acidic solution of isopropanol (90% isopropanol, 10% 1 M HCl in water) for three 4 h intervals. After each interval, the resulting solution was concentrated under vacuum, reconstituted in water and the CSA-131 concentration was determined via LC/MS (using an internal, mass-labelled standard) or HLPC-ELSD. To visualize the coating, coated and uncoated ETT segments (1 cm) were stained with crystal violet (2% w/v in ethanol) for 30 sec, then rinsed with distilled water five times. Segments were dried under vacuum for 1 h. Sections (∼1 mm) were cut and imaged with a light microscope (Axiovert 135, Zeiss, Germany) equipped with a digital camera.
CSA-131 elution studies
Elution from the inner lumen
Coated, intact ETTs (7.5 mm id, 28 cm length) were oriented in a drip flow reactor at a 30˚ angle. The proximal end of the ETT was connected to a peristaltic pump delivering 1 mL/min of sterile PBS. Aliquots of the aqueous solution were collected from the distal end of the ETT in separate vessels at specific time points up to 96 h. The resulting solutions were lyophilized and reconstituted with minimal water prior to CSA-131 concentration determination via LC/MS or HLPC-ELSD.
Elution from the outer lumen
The ends of intact ETTs (7.5 mm id, 28 cm length) were plugged with stoppers, and the tubes were placed in a PVC hose (2.5 cm id, 30 cm length) to approximate the size of an adult trachea. The hose was filled with PBS (120 mL), sealed at the ends with stoppers, and incubated at 37 °C. The resulting solution was collected and replenished with fresh PBS at intervals up to 96 h. CSA-131 concentration was determined as described above.
Efficacy tests
Microbial cultures
Bacterial and fungal cultures were prepared from fresh colonies placed in media and incubated overnight at 37 °C. Pseudomonas aeruginosa (PA01, ATCC 47085), MRSA (BAA-41) and Klebsiella pneumoniae (ATCC 13883) were cultured in trypticase soy broth (TSB). Candida albicans (ATCC 90028) and C. auris (CDC 0383) were cultured in Sabouraud Dextrose Broth (SDB). Bacterial and fungal cultures were pelleted and washed three times in PBS and then resuspended in PBS. Optical density (OD) readings were performed at 600 nm. Aliquots of cultures were diluted in 10% TSB for bacteria or 10% SDB for fungi to a population of 106 cfu/mL and 103 cfu/mL, respectively.
Determination of antimicrobial properties of coated ETTs
Coated ETTs were cut to 5 mm segments and placed in sterile culture tubes. Inoculation solutions were prepared containing 106 cfu/mL (bacteria) or 103 cfu/mL (fungi), and these solutions (1 mL) were used to immerse ETT segments. Tubes were incubated at 37 °C for 24 h. Microbial growth was confirmed when the surrounding growth medium gave a turbidity greater than that of a 0.5 McFarland standard or when less than a 3 log reduction was observed relative to controls with uncoated ETT segments. The latter measurement was made by removing aliquots (10 μL) from the growth media, serially diluting the aliquots, plating them on nutrient agar (TSA for bacteria and SDA for fungi), incubating for 24 h at 37 °C with bacteria and 48 h at 35 °C for fungi and counting resulting colonies. All ETT segments were tested independently in triplicate with uncoated ETT segments used as controls.
Quantification of biofilm growth
Coated ETT segments (5 mm) were treated as described above. For evaluation with mixed-species inocula, bacterial cultures (106 cfu/mL) were mixed (0.5 mL of each) before addition of ETT segments, or a fungal culture (103 cfu/mL, 0.5 mL) was mixed with a bacterial culture (106 cfu/mL, 0.5 mL) before addition of ETT segments. At 24 h intervals, ETT segments were removed (three at each time point), gently rinsed with PBS to remove planktonic organisms, immersed in neutralizing buffer (1 mL, Dey-Engley, Sigma–Aldrich) and sonicated [bath sonicator, Fisher Scientific (FS60), 42 kHz, 100 W] for 15 min.32 The resulting solutions were serially diluted and plated on agar (TSA for bacteria and SDA for fungi), incubated for 24 or 48 h at 35 or 37 °C and colonies were counted.
Scanning electron microscopy (SEM) of microbial biofilms
ETT segments (1 cm) were incubated with bacterial and fungal cultures (1 mL) as described above for the indicated time periods. Controls were prepared by incubating uncoated ETT segments with the same cultures for the same time periods. After incubation, segments were gently washed with Sorensen buffer (0.1 M, pH 7.2) then fixed in 2.5% (w/v) glutaraldehyde in Sorensen buffer at 4 °C overnight, rinsed with Sorensen buffer, immersed in an osmium tetroxide solution (0.5 mL) for 2–3 h and washed with Sorensen buffer to remove excess osmium tetroxide. An ethanol series from 10% to 100% and hexamethyldisilazane were used to dehydrate the samples, which were exposed to air in a desiccator at room temperature for 24 h. Samples were sputter-coated with 5–10 nm of a gold-palladium alloy, and surfaces of a 1 cm section of ETT were visualized under an SEM (FEI Helios NanoLab 600 SEM/FIB, Hillsboro, Oregon, USA).
Measurement of endotoxin levels associated with coated and uncoated ETTs
Endotoxin levels were quantified using a Limulus amoebocyte lysate (LAL) assay with a kinetic chromogenic readout (Charles River Laboratories, Charleston, SC). Assays were performed according to the manufacturer’s instructions with an Escherichia coli endotoxin standard to establish a standard curve. Responses to the endotoxin standard were measured at varied concentrations of CSA-131, and no interference in endotoxin quantification was observed. Uncoated and coated ETT segments (5 mm) were inoculated with P. aeruginosa (PA01, 106 cfu) in TSB and incubated at 37 °C. Aliquots of the fluid were removed at specified times and assayed for endotoxin. At 24 h, tube segments were removed, gently washed with PBS, immersed in neutralizing broth and sonicated (see above) for 15 min. Aliquots of the resulting broth were assayed for endotoxin.
Intubation studies
Six pigs, weighing between 35–45 kg, were intubated. Each animal was anaesthetized and prepped acutely before implantation with a coated or uncoated endotracheal tube followed by monitoring under anaesthesia for 24 h. Blood samples were collected at various time points during the study for pharmacokinetic and cytokine analysis. After the test period, the animals were euthanized and a limited necropsy performed. The trachea, oropharyngeal area, and collateral structures (lungs) were examined for gross abnormalities that may be attributed to the test article.
Ethics
Animal experiment designs were approved by the Institutional Animal Care and Use Committee at Comparative Biosciences, Inc. (Sunnyvale, CA), and experiments were performed under the supervision of this committee.
Results and discussion
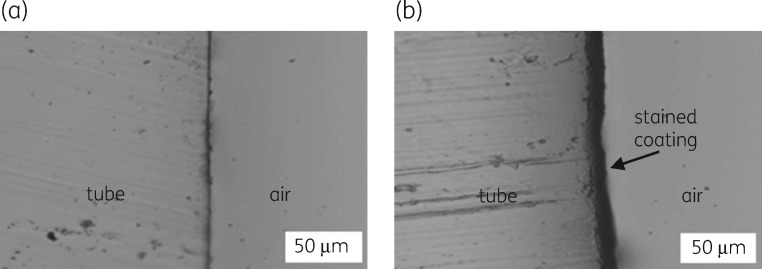
The hydrogel used in the coating is a polyurethane-based, medical-grade, lubricious material developed by Hydromer, Inc. It is generated, after dip coating, via polymerization of isocyanate groups upon exposure to moisture and heat. For clinical use of coated medical devices, sterilization is required, and ETO treatment is the most commonly used method of sterilizing ETTs. Coatings prepared with CSA-131(NDSA) gave a stable coating (13 ± 2 μm) that showed no detectable adduct formation (<1%) by mass spectrometric analysis after ETO treatment. To image the coating, coated and uncoated ETT segments were treated with crystal violet. Coated segments retained the dye, while uncoated segments remained unstained (Figure 2). The stained coating proved uniform with a thickness of approximately 10 μm. The amount of CSA-131(NDSA) added to the coating solution was 10% (w/w) of the non-volatiles in solution. To quantify the amount of ceragenin in the coating, CSA-131 was extracted from coated ETT segments, and from these experiments, we determined that the total amount of CSA-131 was 39.4 μg/cm2 (6.9 mg on the entire tube).
Figure 2.
Microscopy images of the ETT inner lumen stained with crystal violet. (a) Uncoated ETT. (b) Coated ETT.
CSA-131(NDSA) has limited solubility (<20 μg/mL) in water, and for the solubility of CSA-131 to increase in water, ion exchange must occur (e.g. chloride for NDSA). This ion exchange process, as well as the structure of the polyurethane, contributes to the controlled release of the ceragenin from the coating. The rate at which CSA-131 eluted from the coating was determined using two methods. For the inner lumen, a drip flow procedure was used in which PBS was dripped through intact, coated ETTs at the rate of 1 mL/min, the rate at which fluids pass through ETTs in the trachea. For the outer lumen, coated ETTs were incubated in a larger tube of the approximate size of an adult trachea (2.5 cm diameter and 30 cm length). Rates of elution from the inner lumen (drip-flow method) are shown in Table 1; after the first hour of elution, the concentration of CSA-131 dropped below the detection limit (0.25 μg/mL). Elution from the outer lumen was measured over 96 h, and comparable amounts eluted over each 24 h period (Table 1). Notably, in both the drip flow elution and elution into a static solution, the concentration of CSA-131 never exceeded 1 μg/mL.
Table 1.
Elution of CSA-131 from the inner lumen of intact, coated ETTs measured via drip flow (1 mL/min)
| Surface/time interval | Amount eluted (μg) | Elution (μg/cm2) | Concentration of CSA-131 in eluent (μg/mL) |
|---|---|---|---|
| Inner lumena | |||
| 0–30 min | 22.2 | 0.30 | 0.74 |
| 30–60 min | 9.3 | 0.12 | 0.31 |
| 60–120 min | BDLb | NA | NA |
| Totals | 31.5 | 0.42 | |
| Outer lumenc | |||
| 0–24 h | 94.3 | 0.95 | 0.79 |
| 24–48 h | 84.9 | 0.85 | 0.71 |
| 48–72 h | 64.9 | 0.65 | 0.54 |
| 72–96 h | 80.1 | 0.81 | 0.67 |
| Totals | 324 | 3.26 |
Inner lumen elution measured via drip flow (1 mL/min) with an ETT inner lumen surface area of 74.8 cm2.
BDL, below detection limit (0.25 μg/mL).
Outer lumen elution measured in a static solution (120 mL) with an ETT outer lumen surface area of 99.4 cm2.
To determine the efficacy of coated ETTs in preventing microbial growth, bacterial suspensions (106 cfu/mL) or fungal suspensions (103 cfu/mL) were prepared in growth media, and segments (5 mm) of coated ETTs were immersed in the media. The suspensions were incubated for 24 h, and the tube segments were re-challenged every 24 h in fresh growth media. Two measures of microbial growth were quantified: (i) microbial growth in the surrounding medium; and (ii) microbial biofilm formation on the tube segments. For the first measure, aliquots were removed from the growth media, serially diluted and plated; with this measure, continuous testing was possible. In contrast, to quantify biofilm growth, it was necessary to remove tube segments, gently wash them in PBS and sonicate them in neutralizing broth. Tube segments thus treated could not be returned for continued study because sonication may have altered the structure of the coating.
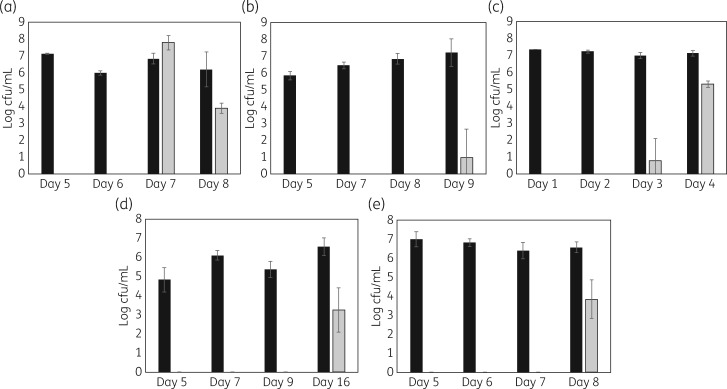
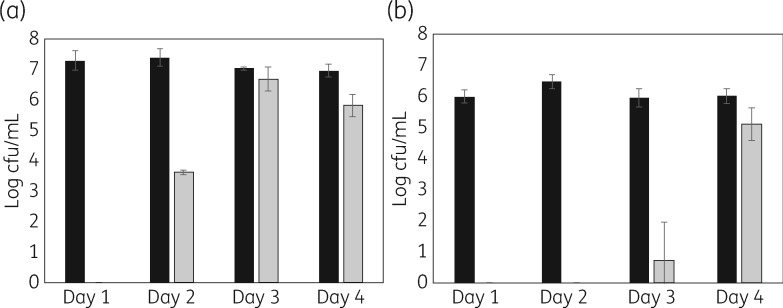
As anticipated, with uncoated tube segments, microbial growth was observed in the growth media and biofilms formed on the segments. In contrast, coated ETT segments prevented microbial growth in the surrounding media and prevented biofilm formation for multiple days. Quantification of microbial biofilm formation on tube segments with representative organisms is shown in Figure 3. Duration of activity ranged from 4 days with P. aeruginosa to 16 days with C. albicans. During these intervals, biofilm formation was reduced by ∼6 log, with no bacteria or fungi detected on the tube segments. Because colonization of ETTs is polymicrobial, we also quantified microbial growth from mixed species inocula (MRSA with PA01 and PA01 with C. auris). While the duration of prevention of biofilm formation decreased in the presence of mixed species of micro-organisms (Figure 4), the coated tubes continued to reduce or prevent colonization for multiple days.
Figure 3.
Biofilms formed on ETT segments (5 mm) after the indicated number of days of incubation (daily exchange of growth medium and re-inoculation). Measurements were made in triplicate. Black bars, uncoated tube segments; grey bars, coated segments. (a) MRSA (BAA-41, 106 cfu inoculation). (b) K. pneumoniae (ATCC 13883, 106 cfu inoculation). (c) P. aeruginosa (ATCC 47085, 106 cfu inoculation). (d) C. albicans (ATCC 90028, 103 cfu inoculation). (e) C. auris (CDC 0383, 103 cfu inoculation).
Figure 4.
Mixed species biofilms formed on ETT segments (5 mm) after the indicated number of days of incubation (daily exchange of growth medium and re-inoculation). Measurements made in triplicate. Black bars: uncoated tube segments; gray bars: coated segments. (a) MRSA (BAA-41, 106 cfu inoculation) with P. aeruginosa (ATCC 47085, 106 cfu inoculation). (b) P. aeruginosa (ATCC 47085, 106 cfu inoculation) with C. auris (CDC 0383, 103 cfu inoculation).
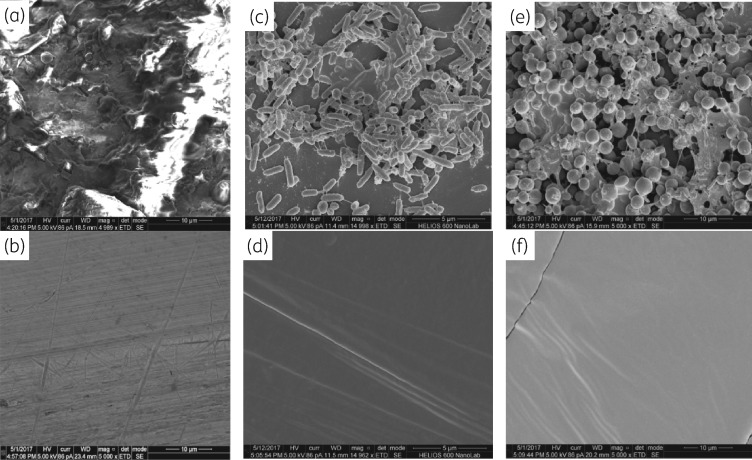
To visualize biofilm formation and prevention thereof, we prepared samples for SEM. Representative images are shown in Figure 5. C. auris (CDC 383) readily formed a stable biofilm on uncoated ETT segments even though this organism has been reported to form less biofilm than C. albicans.33 After 14 days of growth (with re-inoculation every 24 h), the fungal biofilm was nearly completely covered with the extracellular matrix (Figure 5a), seen as a ‘cotton-like mass’ with C. albicans biofilm formation.34 At 14 days, the coated ETT surface showed no fungal growth by SEM (Figure 5b). Culturing of adhered organisms indicated fungal colonization of coated tubes by day 8 (approximately a 3 log reduction relative to controls, Figure 3); however, the populations of fungi adhered to coated tubes did not reach the levels of those adhered to uncoated tubes through 14 days. The lack of fungi observed by SEM may be due to loss of loosely adhered organisms on the coated tubes. Mixed species challenge of uncoated ETTs at 48 h with MRSA and PA01 showed biofilm formation in which both types of bacteria were readily distinguishable (Figure 5c), while the coated segment remained uncolonized (Figure 5d). Similarly, challenge of uncoated tube segments with a combination of PA01 and C. auris showed biofilm formation with both organisms (Figure 5e); extensive colonization of Candida biofilms by Pseudomonas has been observed.35 There was no biofilm formation on the coated ETT segment (Figure 5f).
Figure 5.
SEM images of ETT surfaces. (a) Biofilm of C. auris (CDC 383) on an uncoated tube after 14 days (daily inoculation). (b) Surface of a coated tube after 14 days (daily inoculation with C. auris). (c) Mixed species biofilm of MRSA and PA01 on an uncoated tube after 48 h. (d) Surface of a coated tube after 48 h (inoculation with MRSA and PA01). (e) Mixed species biofilm of PA01 and C. auris on an uncoated tube after 48 h. (f) Surface of a coated tube after 48 h (inoculation with PA01 and C. auris).
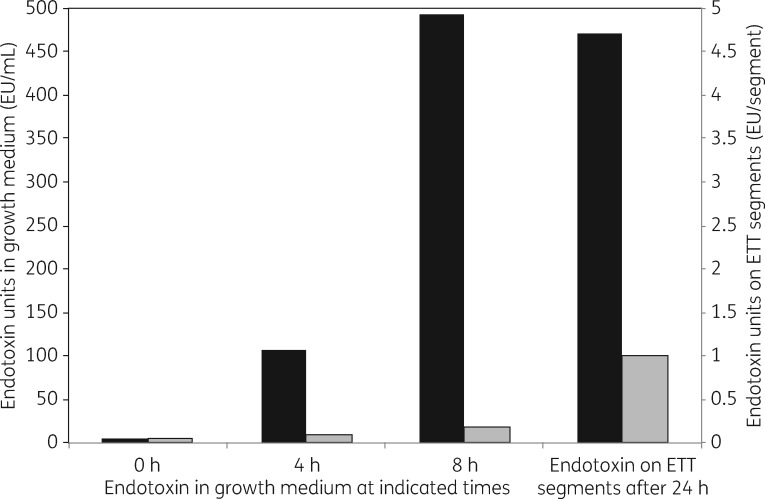
Inflammation, in response to endotoxin, is a primary cause of lung damage and other deleterious responses to ventilator-associated infections.7–11 The antimicrobial effects of the ETT coating were expected to reduce the amount of endotoxin on the tubes and in the surrounding growth media. To test this hypothesis, coated and uncoated ETT segments were exposed to P. aeruginosa (PA01) in a nutrient medium for 24 h. Aliquots from the resulting broth were serially diluted and assayed for endotoxin. To quantify endotoxin on tube surfaces, tubes were removed from culture, gently rinsed with PBS to remove planktonic bacteria and sonicated in neutralizing broth. Endotoxin amounts in the growth medium and on the tube segments are shown in Figure 6. Concomitant with bacterial growth, endotoxin amounts increased in the presence of uncoated tube segments, both on the segments and in the growth medium. In contrast, with the coated segments, endotoxin was substantially reduced in the growth medium and on the tube segments.
Figure 6.
Quantification of the amounts of endotoxin in the growth medium and on ETT segments after incubation with P. aeruginosa for the indicated time periods. Black bars, uncoated tube segments. Grey bars, coated tube segments.
To demonstrate the safety of the coated ETT, a study (Good Laboratory Practice) of intubation in pigs was performed. The animals were intubated and mechanically ventilated for 24 h, and to simulate a worst-case scenario, test samples were manufactured with CSA-131(NDSA) in an amount 133% of the coated ETTs described above. There was no mortality during the study, and the six treated pigs were humanely euthanized according to protocol at their scheduled endpoints of 24 h post treatment. The oropharyngeal area, trachea and lungs were examined for gross abnormalities. There were no significant macroscopic findings in these six animals. Histological analyses were performed on lung and tracheal tissue. The test animals had no inflammation in the lungs while the control animals had minimal inflammation in some sections (data not shown). The interstitial oedema seen in the lungs of both the test and control animals is considered not related to either device but to the procedure and prolonged recumbency of the animals. The test animals had increased cilia loss in the trachea compared with the control animals but it was not a significant difference. In the trachea and in the lung, under the conditions of this study and based on the Irritant Rank score, the test article was considered a non-irritant when compared with the control. To determine if intubation with coated ETTs resulted in systemic exposure to CSA-131, blood samples were taken and analysed for CSA-131 at times between 0 and 24 h post intubation. Both the controls and the test group had no detectable CSA found in the blood (detection limit of 5 ng/mL).
Conclusions
There is a pressing need for development of means of protecting medical devices from microbial colonization, and this need is complicated by the continuous emergence of drug-resistant pathogens. The broad-spectrum antimicrobial activities of the ceragenins, including activity against drug-resistant bacteria and fungi, qualifies them as a potential solution to this need. Use of a ceragenin to protect a medical device required optimization of coating method and composition; full characterization of the coating involved study of ceragenin elution and protection of surfaces from bacterial and fungal challenges; and demonstration of safety in a porcine intubation model. These steps have been completed and the ceragenin-coated ETTs appear well suited for use in preventing microbial colonization and reduction of inflammatory responses to bacteria and fungi in intubated patients.
Acknowledgments
Funding
Generous funding is acknowledged from the National Institutes of Health (R41DK102237), N8 Medical, Inc, CSA Biotechnologies and Brigham Young University.
Transparency declarations
R. B. and C. G. are employees of N8 Medical, Inc., which has licensed fields of the ceragenin technology. P. B. S. is a paid consultant for N8 Medical, Inc. All other authors: none to declare.
References
- 1. Tecle T, Tripathi S, Hartshorn KL.. Defensins and cathelicidins in lung immunity. Innate Immun 2010; 16: 151–9. [DOI] [PubMed] [Google Scholar]
- 2. Lecaille F, Lalmanach G, Andrault PM.. Antimicrobial proteins and peptides in human lung diseases: a friend and foe partnership with host proteases. Biochimie 2016; 122: 151–68. [DOI] [PubMed] [Google Scholar]
- 3. Hotterbeekx A, Xavier BB, Gielen K. et al. The endotracheal tube microbiome associated with Pseudomonas aeruginosa or Staphylococcus epidermis. Sci Rep 2016; 6: 36507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cairns S, Thomas JG, Hooper SJ. et al. Molecular analysis of microbial communities in endotracheal tube biofilms. PLoS One 2011; 6: e14759.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haas CF, Eakin RM, Konkle MA. et al. Endotracheal tubes: old and new. Respir Care 2014; 59: 933–55. [DOI] [PubMed] [Google Scholar]
- 6. Mehta A, Bhagat R.. Preventing ventilator-associated infections. Clin Chest Med 2016; 37: 683–92. [DOI] [PubMed] [Google Scholar]
- 7. Yang YW, Joang YZ, Hsu CM. et al. Pseudomonas aeruginosa ventilator-associated pneumonia induces lung injury through TNF-α/c-Jun NH2-terminal kinase pathways. PLoS One 2017; 12: e0169267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nathe KE, Mancuso CJ, Parad R. et al. Innate immune activation in neonatal tracheal aspirates suggests endotoxin-driven inflammation. Pediatr Res 2012; 72: 203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiesa C, Pacifico L, Natale F. et al. Fetal and early neonatal interleukin-6 response. Cytokine 2015; 76: 1–12. [DOI] [PubMed] [Google Scholar]
- 10. Pelekanou A, Tsangaris I, Kotsaki A. et al. Decrease of CD4-lymphocytes and apoptosis of CD14-monocytes are characteristic alterations in sepsis caused by ventilator-associated pneumonia: results from an observational study. Crit Care 2009; 13: R172.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ely EW, Shintani A, Truman B. et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004; 14: 1753–62. [DOI] [PubMed] [Google Scholar]
- 12. Rodrigues ME, Lopes SP, Pereira CR. et al. Polymicrobial ventilator-associated pneumonia: fighting in vitro Candida albicans-Pseudomonas aeruginosa biofilms with antifungal-antibacterial combination therapy. PLoS One 2017; 12: e0170433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flemming HC, Wingender J, Szewzyk U. et al. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 2016; 14: 563–75. [DOI] [PubMed] [Google Scholar]
- 14. Burmølle M, Ren D, Bjarnsholt T. et al. Interactions in multispecies biofilms: do they actually matter? Trends Microbiol 2014; 22: 84–91. [DOI] [PubMed] [Google Scholar]
- 15. Li J, Rayner CR, Nation RL. et al. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 2006; 50: 2946–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng YH, Lin TL, Pan YJ. et al. Colistin resistance mechanisms in Klebsiella pneumoniae strains from Taiwan. Antimicrob Agents Chemother 2015; 59: 2909–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castanheira M, Griffin MA, Deshpande LM. et al. Detection of mcr-1 among Escherichia coli clinical isolates collected worldwide as part of the SENTRY antimicrobial surveillance program during 2014-2015. Antimicrob Agents Chemother 2016; 60: 5623–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Larkin E, Hager C, Chandra J. et al. The emerging Candida auris: characterization of growth phenotype, virulence factors, antifungal activity, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob Agents Chemother 2017; 61: e02396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stulik L, Hudcova J, Craven DE. et al. Low efficacy of antibiotics against Staphylococcus aureus airway colonization in ventilated patients. Clin Infect Dis 2017; 64: 1081–8. [DOI] [PubMed] [Google Scholar]
- 20. Burnham JP, Kollef MH.. Prevention of Staphylococcus aureus ventilator-associated pneumonia: conventional antibiotics won’t cut it. Clin Infect Dis 2017; 64: 1089–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Griffin M, Kosmisky DE, Templin MA. et al. Antifungal use in immunocompetent, critically ill patients with pneumonia does not improve clinical outcomes. Heart Lung 2016; 45: 538–43. [DOI] [PubMed] [Google Scholar]
- 22. Tokmaji G, Vermeulen H, Müller MCA. et al. Silver-coated endotracheal tubes for prevention of ventilator-associated pneumonia in critically ill patients. Cochrane Database Syst Rev 2015; issue 8: CD009201.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Politano AD, Campbell KT, Rosenberger LH. et al. Use of silver in the prevention and treatment of infections: silver review. Surg Infect (Larchmt) 2013; 14: 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luo Y, McLean DTF, Linden GJ. et al. The naturally occurring host defense peptide, LL-37, and its truncated mimetics KE-18 and KR-12 have selected biocidal and antibiofilm activities against Candida albicans, Staphylococcus aureus, and Escherichia coli in vitro. Front Microbiol 2017; 8: 544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hilchie AL, Wuerth K, Hancock REW.. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol 2013; 9: 761–8. [DOI] [PubMed] [Google Scholar]
- 26. Hashemi MM, Holden BS, Durnas B. et al. Ceragenins as mimics of endogenous antimicrobial peptides. J Antimicrob Agents 2017; 3: 141. [Google Scholar]
- 27. Lai X-Z, Feng Y, Pollard J. et al. Ceragenins: cholic acid-based mimics of antimicrobial peptides. Acc Chem Res 2008; 41: 1233–40. [DOI] [PubMed] [Google Scholar]
- 28. Hashemi MM, Rovig J, Weber S. et al. Susceptibility of colistin-resistant, Gram-negative bacteria to antimicrobial peptides and ceragenins. Antimicrob Agents Chemother 2017; 61: e00292-17.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vila-Farrés X, Callarisa AE, Gu X. et al. CSA-131, a ceragenin active against colistin-resistant Acinetobacter baumannii and Pseudomonas aeruginosa clinical isolates. Int J Antimicrob Agents 2015; 46: 568–71. [DOI] [PubMed] [Google Scholar]
- 30. Durnas B, Wnorowska U, Pogoda K. et al. Candidacidal activity of selected ceragenins and human cathelicidin LL-37 in experimental settings mimicking infections sites. PLoS One 2016; 11: e0157242.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bucki R, Sostarecz AG, Byfield FJ. et al. Resistance of the antibacterial agent ceragenin CSA-13 to inactivation by DNA of F-actin, and its activity in cystic fibrosis sputum. J Antimicrob Chemother 2007; 60: 535–45. [DOI] [PubMed] [Google Scholar]
- 32. Beyth N, Bahir R, Matalon S. et al. Streptococcus mutans biofilm changes surface-topography of resin composites. Dent Mater 2008; 24: 732–6. [DOI] [PubMed] [Google Scholar]
- 33. Sherry L, Ramage G, Kean R. et al. Biofilm-forming capacity of highly virulent, multidrug-resistant Candida auris. Emerg Infect Dis 2017; 23: 328–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abd E-BRM, Ela DMMAE, Gad GFM.. N-acetylcysteine inhibits and eradicates Candida albicans biofilms. Am J Infect Dis Microbiol 2014; 2: 122–30. [Google Scholar]
- 35. Fourie R, Ells R, Swart CW. et al. Candida albicans and Pseudomonas aeruginosa interaction with focus on the role of eicosanoids. Front Physiol 2016; 7: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]