Abstract
Background: The second-line drugs recommended to treat drug-resistant TB are toxic, expensive and difficult to procure. Given increasing resistance, the need for additional anti-TB drugs has become more urgent. But new drugs take time to develop and are expensive. Some commercially available drugs have reported anti-mycobacterial activity but are not routinely used because supporting laboratory and clinical evidence is sparse.
Methods: We analysed 217 MDR M. tuberculosis isolates including 153 initial isolates from unique patients and 64 isolates from follow-up specimens during the course of treatment. The resazurin microdilution assay was performed to determine MICs of trimethoprim/sulfamethoxazole, mefloquine, thioridazine, clofazimine, amoxicillin/clavulanate, meropenem/clavulanate, nitazoxanide, linezolid and oxyphenbutazone. Isoniazid was used for validation. We calculated the MIC50 and MIC90 as the MICs at which growth of 50% and 90% of isolates was inhibited, respectively.
Results: The MIC50s, in mg/L, for initial isolates were as follows: trimethoprim/sulfamethoxazole, 0.2/4; mefloquine, 8; thioridazine, 4; clofazimine, 0.25; amoxicillin/clavulanate, 16/8; meropenem/clavulanate, 1/2.5; nitazoxanide, 16; linezolid, 0.25; and oxyphenbutazone, 40. The MIC90s, in mg/L, for initial isolates were as follows: trimethoprim/sulfamethoxazole, 0.4/8; mefloquine, 8; thioridazine, 8; clofazimine, 0.5; amoxicillin/clavulanate, 32/16; meropenem/clavulanate, 8/2.5; nitazoxanide, 16; linezolid, 0.25; and oxyphenbutazone, 60. By comparison, the MIC90 of isoniazid was >4 mg/L, as expected. There was no evidence that previous treatment affected susceptibility to any drug.
Conclusions: Most drugs demonstrated efficacy against M. tuberculosis. When these MICs are compared with the published pharmacokinetic/pharmacodynamic profiles of the respective drugs in humans, trimethoprim/sulfamethoxazole, meropenem/clavulanate, linezolid, clofazimine and nitazoxanide appear promising and warrant further clinical investigation.
Introduction
Drug-resistant TB has become a major public health problem in many nations. When Mycobacterium tuberculosis is resistant to at least isoniazid and rifampicin (known as MDR-TB), it becomes exceedingly difficult to treat, requiring prolonged treatment with multiple second-line drugs that are less effective, much more toxic and expensive, and often difficult to procure.
Despite the urgent need for better treatments, development of new drugs is slow because they must be proved safe in humans. However, many drugs were developed for a specific indication and later proved to be effective for other, unrelated conditions.
Several commercially available drugs have been reported to have anti-mycobacterial activity but are currently not recommended for routine use in the treatment of MDR-TB because supporting laboratory and clinical evidence is considered insufficient.1,2 We sought to determine the in vitro efficacy against MDR M. tuberculosis of nine such drugs, comparing MICs with their reported pharmacokinetic (PK) and pharmacodynamic (PD) properties: trimethoprim/sulfamethoxazole, mefloquine, thioridazine, clofazimine, amoxicillin/clavulanate, meropenem/clavulanate, nitazoxanide, linezolid and oxyphenbutazone.
For each drug, there is evidence of activity against M. tuberculosis, they are widely available and most are relatively inexpensive with known safety profiles; all but oxyphenbutazone are approved by the US FDA. With the exception of linezolid, robust data on their activity against drug-resistant M. tuberculosis are limited, but if additional evidence demonstrates efficacy, they could be re-purposed for MDR-TB, substantially expanding the current armamentarium.
Materials and methods
Study isolates
We selected 228 MDR M. tuberculosis isolates, including 160 isolates that were taken at the beginning of MDR-TB therapy from unique individuals (initial isolates) and 68 isolates from later in the course of MDR-TB therapy from some of those same individuals (follow-up isolates; follow-up range 2–22 months). We purposely selected some isolates from all patients who had previous treatment with medications from the same classes of drugs we tested, with the exception of those who had previously been treated with amoxicillin/clavulanate. Because there were many patients who had been treated with amoxicillin/clavulanate, we selected a simple random sample of those patients. These isolates were part of a US CDC study: the Preserving Effective Treatment of Tuberculosis Study (PETTS), a multinational prospective cohort study that investigated the prevalence of and risk factors for resistance to second-line drugs in patients with MDR-TB.3
Isolates were transferred to Taiwan CDC for drug susceptibility testing. Isolates were subcultured using Middlebrook 7H9 Broth (BBL™ MGIT™ Mycobacteria Growth Indicator Tube, BD, Taipei, Taiwan). We verified pure growth as M. tuberculosis using the p-nitrobenzolic acid inhibition test (Sancordon Inc., Taipei, Taiwan) and checked the growth on a sheep blood agar plate (TSA II™, BD, Taipei, Taiwan) and with a rapid molecular test.
Antimicrobial drugs
Pure powders of linezolid, trimethoprim, sulfamethoxazole, mefloquine, thioridazine, clofazimine, amoxicillin, meropenem and nitazoxanide were obtained from US Pharmacopeia. Powders of isoniazid and clavulanate were from Sigma-Aldrich Chemie GmbH. Oxyphenbutazone powder was purchased from Toronto Research Chemicals, Canada. Trimethoprim and amoxicillin were dissolved in 0.1 N HCl; sulfamethoxazole and mefloquine were dissolved in NaOH and ethanol, respectively; thioridazine, clofazimine, nitazoxanide, meropenem and oxyphenbutazone were dissolved in DMSO; and clavulanate, isoniazid and linezolid were dissolved in sterile deionized water (maximum solvent concentrations: 0.7% HCl, 1.3% NaOH, 6.4% ethanol, 2.5% distilled water, 2.0% DMSO). Stock solutions were sterilized using 0.22 μm filters and stored in a −80°C freezer.
Drug susceptibility testing
We tested each isolate against the following drugs: trimethoprim/sulfamethoxazole, mefloquine, thioridazine, clofazimine, amoxicillin/clavulanate, meropenem/clavulanate, nitazoxanide, linezolid and oxyphenbutazone. We tested the isolates against isoniazid as a form of control and validation, and to test for differences between patients who had and had not been treated with isoniazid previously. We determined MICs using a broth microdilution method performed in 96-well plates according to a previously reported resazurin microdilution assay (REMA) protocol.4,5 We used two plates for testing each isolate. The plates were arrayed as eight rows and 12 columns, and we used a different row for each drug, and tested increasing concentrations of each drug across the columns (Table 1).
Table 1.
Layout of drugs and their concentrations in each pair of 96-well plates
| Column 1 | Column 2 | Column 3 | Column 4 | Column 5 | Column 6 | Column 7 | Column 8 | Column 9 | Column 10 | Column 11 | Column 12 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plate 1 | SDW | SDW | SDW | SDW | SDW | SDW | SDW | SDW | SDW | SDW | SDW | SDW | |
| NTZ | SDW | 128 | 64 | 32 | 16 | 8 | 4 | 2 | 1 | GC (+) | GC (−) | SDW | |
| MXFa | SDW | 2 | 1 | 0.5 | 0.25 | 0.12 | 0.06 | 0.03 | 0.015 | GC (+) | GC (−) | SDW | |
| SXT | SDW | 7/128 | 3.5/64 | 1.7/32 | 0.8/16 | 0.4/8 | 0.2/4 | 0.1/2 | 0.05/1 | GC (+) | GC (−) | SDW | |
| INH | SDW | 4 | 2 | 1 | 0.5 | 0.25 | 0.12 | 0.06 | 0.03 | GC (+) | GC (−) | SDW | |
| LZD | SDW | 8 | 4 | 2 | 1 | 0.5 | 0.25 | 0.12 | 0.06 | GC (+) | GC (−) | SDW | |
| SDW | SDW | ||||||||||||
| SDW | SDW | SDW | SDW | SDW | SDW | SDW | SDW | SDW | SDW | SDW | SDW | ||
| Plate 2 | SDW | SDW | SDW | SDW | SDW | SDW | SDW | SDW | SDW | SDW | SDW | SDW | |
| OPB | SDW | 90 | 80 | 70 | 60 | 50 | 40 | 30 | 20 | GC (+) | GC (−) | SDW | |
| MEM/CLA | SDW | 16/2.5 | 8/2.5 | 4/2.5 | 2/2.5 | 1/2.5 | 0.5/2.5 | 0.25/2.5 | 0.12/2.5 | GC (+) | GC (−) | SDW | |
| MEF | SDW | 64 | 32 | 16 | 8 | 4 | 2 | 1 | 0.5 | GC (+) | GC (−) | SDW | |
| AMC | SDW | 64/32 | 32/16 | 16/8 | 8/4 | 4/2 | 2/1 | 1/0.5 | 0.5/0.25 | GC (+) | GC (−) | SDW | |
| CLO | SDW | 4 | 2 | 1 | 0.5 | 0.25 | 0.12 | 0.06 | 0.03 | GC (+) | GC (−) | SDW | |
| TDZ | SDW | 128 | 64 | 32 | 16 | 8 | 4 | 2 | 1 | GC (+) | GC (−) | SDW | |
| SDW | SDW | SDW | SDW | SDW | SDW | SDW | SDW | SDW | SDW | SDW | SDW | ||
NTZ, nitazoxanide; MXF, moxifloxacin; SXT, trimethoprim/sulfamethoxazole; INH, isoniazid; LZD, linezolid; OPB, oxyphenbutazone; MEM/CLA, meropenem/clavulanate; MEF, mefloquine; AMC, amoxicillin/clavulanate; CLO, clofazimine; TDZ, thioridazine; GC, growth control; (+), bacilli and drug-free medium; (−), medium only; SDW, sterilized deionized H2O.
All drug concentrations are in mg/L.
Data not included in this article.
We grew isolates to a uniform OD with a turbidity equivalent to that of a 1.0 McFarland standard in 7H9-S broth, and diluted them 1:10. We diluted each drug, except oxyphenbutazone, at four times the highest desired drug concentration and prepared the plates according to protocol. For oxyphenbutazone, we developed a working solution that was twice the desired concentration for each well, and added that to an equal volume of 7H9 broth; potassium dihydrogen phosphate (KH2PO4) was added to 7H9 broth to reach a pH of 5.9–6.0. We added culture dilution to each well containing drug, and followed protocol for incubation and reading.4 For determination of the quality control range of each drug, we tested 25 aliquots of pan-susceptible H37Rv (ATCC® 27294™) reference strain against the same concentrations of drugs using the same protocols. To determine the effect of the solvent on MIC determination, we assessed the effect of drug-free solvent on H37Rv before testing the dissolved drugs on the study isolates.
Data analysis
According to protocol, the MIC was defined as the lowest drug concentration that prevented the pink colour change.4 Plates were read by two medical technologists independently, and any discordant readings were adjudicated by a third technologist. We defined the MIC50 and MIC90 as the MICs that inhibited growth of 50% and 90% of isolates, respectively. We treated the MIC values as ordinal, and evaluated the differences between MIC values for initial isolates from patients previously treated with a drug from a class which we were testing to patients without previous treatment with a drug from that class. We also evaluated the differences in MIC values between follow-up isolates from patients who were treated with a drug class we were testing during MDR-TB therapy and isolates from different patients who were not treated with a drug from that class. Because MICs were recorded as discrete ordinal values (i.e. not continuous) and were not reliably normally distributed, and numbers of isolates were small, we used the exact Wilcoxon–Mann–Whitney test.
This protocol, and all study activities, were reviewed and approved by Institutional Review Board (IRB) C at the US CDC. This study was also approved by IRB at Taiwan CDC (TwCDCIRB102028).
Results
Of the 228 MDR M. tuberculosis isolates, 7 were contaminated, 1 had no growth and 3 did not have enough bacilli to determine the MICs, which left 217 isolates for analysis. Of these, 153 were initial isolates from unique patients and 64 were follow-up isolates obtained in the course of treatment.
MICs
The MICs of the solvents alone for the H37Rv strain were 14% DMSO, >8% ethanol, >8% NaOH and >8% HCl.
Initial isolates
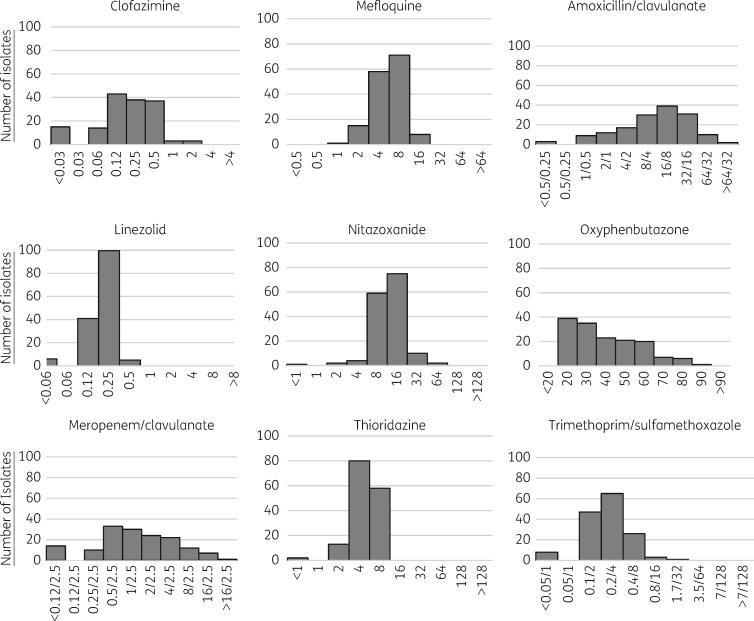
Frequency histograms of the MICs of each of the tested drugs are shown in Figure 1. MIC90 and MIC50 values of each drug are shown in Table 2, alongside the MIC90 values for the H37Rv controls. The range of MICs of each of the drugs varied widely, but had the least variability for trimethoprim/sulfamethoxazole, mefloquine, thioridazine and linezolid. The drugs with documented serum peak levels above the MIC90 were trimethoprim/sulfamethoxazole, nitazoxanide, meropenem/clavulanate and linezolid (Tables 2 and 3).
Figure 1.
MICs, in mg/L, of various antibiotics for initial MDR M. tuberculosis isolates; n = 153. One hundred and fifty initial isolates had valid results for trimethoprim/sulfamethoxazole and 152 had valid results for oxyphenbutazone.
Table 2.
MICs, in mg/L, for initial isolates and control H37Rv
| Drug | Initial isolates, n = 153 |
Control H37Rv, n = 25 | ||
|---|---|---|---|---|
| MIC50 | MIC90 | MIC range | MIC90 (MIC range) | |
| Trimethoprim/sulfamethoxazolea | 0.2/4 | 0.4/8 | 0.05/1–1.7/32 | 0.2/4 (0.1/2–0.4/8) |
| Mefloquine | 8 | 8 | 1–16 | 8 (4–8) |
| Thioridazine | 4 | 8 | <1–8 | 8 (4–8) |
| Clofazimine | 0.25 | 0.5 | <0.03–2 | 0.25 (0.125–0.5) |
| Amoxicillin/clavulanate | 16/8 | 32/16 | <0.5/0.25 to >64/32 | 32/16 (16/8–32/16) |
| Meropenem/clavulanate | 1/2.5 | 8/2.5 | <0.12/2.5 to >16/2.5 | 2/2.5 (2/2.5–4/2.5) |
| Nitazoxanide | 16 | 16 | <1–64 | 16 (8–32) |
| Linezolid | 0.25 | 0.25 | <0.06–0.5 | 0.5 (0.25–0.5) |
| Oxyphenbutazoneb | 40 | 60 | 20 to >80 | 60 (40–90) |
| Isoniazidb | 4 | >4 | <0.03 to >4 | <0.03 (<0.03–0.06) |
A total of 150 isolates had valid results for trimethoprim/sulfamethoxazole.
A total of 152 isolates had valid results for oxyphenbutazone and isoniazid.
Table 3.
PK properties of tested drugs
| Drug | Time versus concentration dependent | Serum peak | AUC | References |
|---|---|---|---|---|
| Trimethoprim/ sulfamethoxazole | both | 52 (free) and 68 (total) mg/L at steady-state for 160/800 mg twice-daily dosing | 471 mg·h/L steady-state to 800 mg daily dose | http://www.accessdata.fda.gov/drugsatfda_docs/label/2003/17377slr057_Bactrim_lbl.pdf; Winter et al.69 |
| Mefloquine | concentration | 1.20–1.60 mg/L at steady-state for 250 mg weekly dosing | 645 mg·h/L (post-prandial) after single 750 mg dose | http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/019591s023lbl.pdf; Wernsdorfer et al.;20 Karbwang et al.;70 Crevoisier et al.71 |
| Amoxicillin/ clavulanate | time | 17.0 mg/L after single post-prandial dose of 2000 mg of AUGMENTIN XR | 71.6 mg·h/L after single post-prandial dose of 2000 mg of AUGMENTIN XR | http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050785s007lbl.pdf |
| Clofazimine | concentration | 0.369 mg/L after single 200 mg dose administered with anti-TB medicines and a high-fat meal; 1.0 mg/L average serum concentration for 300 mg daily dosing | 3.690 mg·h/L after single 200 mg dose administered with anti-TB medicines and a high-fat meal | http://www.accessdata.fda.gov/drugsatfda_docs/label/2003/19500slr010_lamprene_lbl.pdf; Nix et al.;28 Xu et al.;72 Steel et al.73 |
| Thioridazine | undetermined | 0.3718 mg/L after single 100 mg dose | 2.603 mg·h/L after single 100 mg dose | Chakraborty et al.74 |
| Nitazoxanide | both | tizoxanide: 10.6 mg/L; tizoxanide-gluconurate: 10.5 mg/L after single 500 mg dose and tizoxanide: 9.05 mg/L; tizoxanide-gluconurate: 12.2 mg/L after repeated doses of 500 mg twice daily | tizoxanide: 41.9 mg·h/L; tizoxanide-gluconurate: 63.0 mg·h/L after single 500 mg dose | http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021818lbl.pdf; Stockis et al.;54 Lam et al;55 Stockis et al.75 |
| Meropenem/ clavulanate | time | 112 mg/L after 1 g intravenous bolus administered over 5 min | 28.6 mg·h/L for 8 h | http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050706s022lbl.pdf; Li et al.;76 Nicolau77 |
| Oxyphenbutazone | undetermined | |||
| Linezolid | time (concentration enhanced) | 21.2 mg/L with every 12 h dosing 600 mg | 138 mg·h/L with every 12 h dosing 600 mg | http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021130s032,021131s026,021132s031lbl.pdf |
Follow-up isolates of MDR-TB cases treated with certain drugs
Among the follow-up isolates, there was no difference in MICs of drugs for isolates from patients who were and were not previously treated with a drug from the same class. In the comparison of follow-up isolates from patients who were and were not treated with a particular drug during MDR-TB therapy, we noted that isolates from patients treated with clofazimine had apparently lower MICs of clofazimine than those who were not treated with the drug (Table 4).
Table 4.
MICs, in mg/L
| Of specific drugs for initial isolates from patients who did and did not have a history of treatment with a drug from that class, n = 153 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | MIC50 | MIC90 | MIC range | Pa | |||||||
| Amoxicillin/clavulanate for isolates from patients previously treated with β-lactams, n = 2 | NA | NA | 2, 8 | 0.25 | |||||||
| Amoxicillin/clavulanate for isolates from patients not previously treated with β-lactams, n = 151 | 16 | 32 | 0.25–128 | ||||||||
| Isoniazid for isolates from patients previously treated with isoniazid, n = 141b | 4.0 | >4 | <0.03 to >4 | 0.19 | |||||||
| Isoniazid for isolates from patients not previously treated with isoniazid, n = 11b | >4 | >4 | 1 to >4 | ||||||||
| Trimethoprim/sulfamethoxazole for isolates from patients previously treated with trimethoprim/sulfamethoxazole, n = 51c | 0.2/4 | 0.4/8 | <0.05/1–1.7/32 | 0.17 | |||||||
| Trimethoprim/sulfamethoxazole for isolates from patients not previously treated with trimethoprim/sulfamethoxazole, n = 99c | 0.2/4 | 0.4/8 | <0.05/1–0.8/16 | ||||||||
| Of specific drugs for isolates from patients who were and were not treated with a drug from that class during MDR-TB treatment, n = 64 | |||||||||||
| Drug |
Initial isolates |
Follow-up isolates |
|||||||||
| MIC50 | MIC90 | MIC range | MIC50 | MIC90 | MIC range | Pa | |||||
| Amoxicillin/clavulanate for isolates from patients treated with amoxicillin during MDR-TB therapy, n = 33 | 16 | 64 | 1–32 | 16 | 32 | 1–32 | 0.13 | ||||
| Amoxicillin/clavulanate for isolates from patients not treated with amoxicillin during MDR-TB therapy, n = 31 | 8 | 32 | 0.25–32 | ||||||||
| Clofazimine for isolates from patients treated with clofazimine during MDR-TB therapy, n = 3 | NA | NA | 0.05, 0.12, 0.50 | NA | NA | 0.12, 0.12, 0.06 | 0.05 | ||||
| Clofazimine in isolates from patients not treated with clofazimine during MDR-TB therapy, n = 61 | 0.25 | 1.0 | 0.015–2.0 | ||||||||
| Trimethoprim/sulfamethoxazole for isolates from patients treated with trimethoprim/sulfamethoxazole during MDR-TB therapy, n = 15 | 0.2/4 | 0.4/8 | 0.1/2–0.8/16 | 0.2/4 | 0.2/4 | 0.1/2–0.4/8 | 0.40 | ||||
| Trimethoprim/sulfamethoxazole for isolates from patients not treated with trimethoprim/sulfamethoxazole during MDR-TB therapy, n = 49 | 0.2/4 | 0.2/4 | <0.05/1–3.5/64 | ||||||||
NA, not applicable.
P values were determined using the exact Wilcoxon–Mann–Whitney test, and in the top part of the table reflect the differences between isolates from patients with and without a history of treatment with the drug in question, and in the bottom part of the table reflect the differences between patients who were treated and patients who were not treated with the drug in question during MDR-TB therapy.
A total of 152 isolates had valid results for isoniazid.
A total of 150 isolates had valid results for trimethoprim/sulfamethoxazole.
Discussion
This study represents a large panel of MDR M. tuberculosis isolates tested for susceptibility to a variety of commercially available but unconventional drugs; our findings provide compelling data upon which to base recommendations about further clinical investigation. Of the drugs we tested, linezolid has a sufficient evidence base to support recommendations for a clinical susceptibility breakpoint,6 and our data may be helpful in refining it. The other drugs have not been rigorously tested for clinical efficacy against M. tuberculosis, and therefore critical concentrations and PK/PD parameters specific to TB have not been established. Our data can be used to help assess the feasibility of their use against M. tuberculosis by comparing MICs with achievable serum and tissue levels of the antibiotic.
Trimethoprim/sulfamethoxazole
Among existing drugs that could easily be repurposed for treatment of MDR-TB, trimethoprim/sulfamethoxazole is one of the most widely available and best studied. Our MIC data are comparable to what has been recently published in other studies, and validate conclusions made from those data.7–13 The MICs that we documented were well within the steady-state mean plasma level for both free and total sulfamethoxazole, which has been demonstrated to be the active agent against M. tuberculosis, when it is dosed at 800 mg twice daily (Table 3). MICs for initial and follow-up isolates were similar in patients treated with trimethoprim/sulfamethoxazole, which is consistent with previous studies that have not demonstrated acquisition of resistance.9,14 The bactericidal effect of sulfamethoxazole is thought to be both time and concentration dependent; consequently, the free ratio of AUC0–24:MIC is considered an appropriate parameter to predict successful treatment. Although one study has demonstrated that a ratio >25 best predicts the effectiveness of sulfamethoxazole against melioidosis,15 the threshold for this parameter against TB has not been established. Only one study has investigated the PK/PD properties of trimethoprim/sulfamethoxazole against drug-resistant M. tuberculosis, and found that the free AUC0–24:MIC ranges widely: from 3 to 29 among nine patients treated with the relatively low dose of 400 mg of sulfamethoxazole daily.7 Using the AUC0–24 that has been previously documented for 800 mg daily (471 mg·h/L) and the MIC90 (8 mg/L) from our study, the ratio is well over 50. Other studies have suggested a breakpoint of 2/38 mg/L for trimethoprim/sulfamethoxazole;9,16 all of our isolates would be considered susceptible at that concentration. These findings should encourage focused clinical research on this drug. An ongoing clinical investigation (NCT01832987) should provide data on the clinical use and role of trimethoprim/sulfamethoxazole in treating drug-resistant TB.17
Mefloquine
Limited data on the in vitro activity of mefloquine against M. tuberculosis have documented MICs ranging from 8 to 16 mg/L,18,19 and our study validates these findings. Serum levels of mefloquine range from 1 to 2 mg/L at standard dosing for malaria (Table 3), which is well below the demonstrated MICs. But studies have indicated that mefloquine concentrates in macrophages to levels above the MICs.18,20 If this finding can be substantiated, then mefloquine may have clinical utility in the treatment of drug-resistant TB, and further clinical investigation would be warranted.
Thioridazine
Thioridazine inhibited mycobacterial growth at concentrations of 4–8 mg/L, which is similar to what has been previously reported.21,22 Thioridazine exhibits linear kinetics within the dose range used clinically, but serum levels exhibit wide inter-subject variability and are influenced by age, smoking and polymorphisms of metabolic enzymes.23,24 Achievable serum concentrations range between 1 and 21 ng/mL per mg of oral dose,23 and a standardized 100 mg dose correlated with a mean serum level of 0.3718 mg/L (Table 3). High serum levels of thioridazine are potentially toxic, and no studies have reported levels >2 mg/L, which seem to be required to kill extracellular M. tuberculosis. But previous research has indicated that thioridazine accumulates in macrophages, and is bactericidal to intracellular organisms at serum levels correlated to conventional dosing.22 While clinical data on the use of thioridazine for drug-resistant TB are equivocal,25 there remains a potential role for thioridazine, especially among patients requiring treatment for psychosis, as can be induced by cycloserine, or insomnia, as can be induced by fluoroquinolones. The use of thioridazine should be further investigated with additional clinical and PK/PD studies that measure intracellular levels and activity.
Clofazimine
Previous studies have demonstrated that clofazimine has MICs for M. tuberculosis that typically range from 0.125 to 1.0 mg/L.26,27 Our data are comparable, with an MIC90 of 0.5 mg/L. The Cmax of clofazimine ranges widely with dose. The PK/PD literature reports a Cmax of 0.369 mg/L after a single dose of 200 mg (Table 3),28 and average plasma levels of 0.7, 1.0 and 1.41 mg/L that correspond to daily doses of 100, 300 and 400 mg respectively; values as high as 4 mg/L have been reported with 600 mg daily dosing.29,30 A human study failed to show an early bactericidal effect of clofazimine after 2 weeks of therapy,26 but data from animal models suggest that clofazimine concentrates slowly in the lungs and macrophages, reaching very high levels, with an observable clinical effect only after a few weeks of therapy.31–33 Animal studies have found that clofazimine has the ability to shorten duration of treatment,34 and clofazimine has recently been shown to improve clinical markers and endpoints in humans with a safety profile comparable to first-line therapy.35 It is an integral part of the new, shorter regimen for MDR-TB that is being incorporated and studied around the world (http://www.who.int/tb/publications/Short_TB_regimens.pdf). Previous studies have recommended clofazimine breakpoints that range from 0.25 to 1 mg/L;27,31 as clofazimine becomes increasingly used for drug-resistant TB, it will be necessary to establish interpretative criteria for in vitro susceptibility testing correlated with PK/PD and clinical data that account for its intracellular accumulation and clinical effect over time. Our finding that isolates from patients treated with clofazimine had apparently lower MICs of clofazimine than isolates from patients not treated with clofazimine is likely a statistical anomaly, given the small number of isolates (Table 4).
Amoxicillin/clavulanate
Mycobacteria are intrinsically resistant to β-lactams because they produce β-lactamase constitutively, have a relatively impermeable cell wall, and have active efflux transporters.36 Clavulanate inhibits β-lactamase, permitting the bactericidal activity of β-lactam agents.37 Some clinical data have demonstrated bactericidal activity of amoxicillin/clavulanate,38 and laboratory studies have investigated the efficacy of amoxicillin/clavulanate against drug-resistant M. tuberculosis, with variable results.39–42 Our results are comparable to those that demonstrated higher MICs. Bacterial killing by β-lactams is time dependent, and it has been proposed that the best marker for bactericidality is the length of time that serum or tissue levels remain above the MIC. For penicillins in general, a free serum concentration above the MIC for at least 40% of the dosing interval predicts bacterial eradication.43 The sustained release formulation of 2000 mg of amoxicillin/clavulanate is associated with a prolonged free AUC and a Cmax of about 17 mg/L (Table 3); it is not known to concentrate in the lungs or phagocytes.44,45 Our data, documenting an MIC90 of amoxicillin of 32 mg/L, suggests that this dosage of amoxicillin/clavulanate will not be highly efficacious in the treatment of drug-resistant TB. In vitro mouse models have suggested the same.41 There are other data, however, that suggest a synergistic effect between amoxicillin/clavulanate and meropenem,42 and this merits further investigation.
Meropenem/clavulanate
Meropenem has previously been investigated in laboratory studies of drug-resistant isolates of M. tuberculosis,46–48 and our results, with an MIC90 of 8 mg/L, are comparable to investigations that have looked at the combination of meropenem/clavulanate. Meropenem can achieve a high Cmax after intravenous administration (Table 3), substantially higher than the MICs that we documented. But with a reported half-life of 1 h, two to three times daily dosing would be required to maintain serum levels persistently above MICs. Small clinical trials have produced in vivo evidence of the effectiveness of intravenous meropenem/clavulanate,49,50 but the frequency of dosing would probably require inpatient management. A case series documenting the clinical effects of novel regimens that contain ertapenem, a newer carbapenem compound that requires once daily dosing, demonstrated impressive results.51 Our results support additional clinical investigation.
Nitazoxanide
The activity of nitazoxanide against laboratory and clinical strains of M. tuberculosis has been investigated previously,52,53 and is thought to be both dose and time dependent; in previous studies, no evidence of resistance was observed. Our findings are directly comparable to those previously reported, with an MIC90 of 16 mg/L. The plasma levels of nitazoxanide are not measurable, but levels of the two main metabolites, tizoxanide and tizoxanide-gluconurate, can be measured. While there are no recommended PK/PD parameters for nitazoxanide, the combined plasma Cmax levels at standard dosing (500 mg twice daily) of the two measurable metabolites exceed the MICs reported here (Table 3). Nitazoxanide has also been given safely at higher doses (up to 2000 mg twice daily), and has boosted levels when given in a fed state.54 Furthermore, research has suggested that nitazoxanide works in vivo in various ways: by a direct bactericidal effect, by inhibiting regulation of cell metabolism and by inducing autophagy at standard dosing;55 this latter mechanism may render nitazoxanide even more effective in vivo than in vitro.
Linezolid
A growing volume of laboratory data on the effect of linezolid against drug-resistant M. tuberculosis has been documented, with virtually all studies demonstrating efficacy.39,48,56–58 The effect of linezolid is time dependent, and the bactericidal effect of linezolid is thought to be best indicated by either the time serum levels remain above the MIC or a high free AUC:MIC. The free AUC:MIC ratio recommended against Staphylococcus aureus is >80,59 but a comparable value has not been determined for activity against M. tuberculosis. The MICs we documented are well below serum levels that are easy to maintain, and below the proposed critical concentration recommended for DST of M. tuberculosis on agar (1 mg/L).27,48 Given the compelling laboratory data and some observational studies, linezolid has moved into clinical use, and has been increasingly used for the treatment of drug-resistant TB. Findings suggest improved outcomes, but with a notable rate of adverse events.25,60 Given the laboratory efficacy, some have recommended clinical investigation of lower doses in the treatment of TB to diminish adverse events;61 our data support these recommendations. Additional clinical efficacy and PK/PD data will help refine laboratory indicators of clinical success.
Oxyphenbutazone
We were prompted to test isolates of M. tuberculosis against oxyphenbutazone by a recent study that reported no effect of oxyphenbutazone in liquid culture, but low MICs when tested on agar plates (10 mg/L).62 That study documented mycobactericidal activity of oxyphenbutazone against only non-replicating organisms. In a mildly acidic environment (pH 5.0), oxyphenbutazone was readily metabolized to 4-OH-oxyphenbutazone, which was mycobactericidal to both non-replicating and replicating organisms. We found that oxyphenbutazone had a wide range of MICs when testing was conducted at a pH of 6.1, with an MIC90 of 60 mg/L. This extends the previous work, demonstrating in vitro efficacy of this agent in less acidic environments. PK/PD data on oxyphenbutazone and other non-steroidal anti-inflammatory drugs are limited, and higher doses can be harmful; additional research is needed to determine the clinical utility of these agents.63
Isoniazid
As the value of isoniazid for treating TB is well established, it was included in the study primarily because of interest in the meaning of susceptibility or resistance at multiple critical concentrations. Critical concentrations for isoniazid have been established for both low- and high-level resistance and our data clearly demonstrate the anticipated resistance.64 We observed that among the isolates defined as resistant according to established criteria (0.2 mg/L on agar plates), a remarkable 50% had an MIC of ≤4.0 mg/L. Serum levels above this are readily achieved with high-dose isoniazid,65 suggesting that a large proportion of MDR-TB patients may benefit. This is consistent with reports demonstrating the effectiveness of shorter regimens that included moderately high-dose isoniazid.66–68
Limitations
Our findings are limited to laboratory data of isolates, generally derived from second-generation subcultures, the populations of which may have varied from the original. Because we do not have correlating PK/PD data on the various drugs we investigated, we cannot reach conclusions about the clinical use of these drugs. Furthermore, laboratory data using REMA for these drugs are limited and the assay has not been extensively validated for these drugs; the recommendations we make are extrapolations from previous data demonstrating the correlations between REMA and the reference method, and it is uncertain how applicable they are to these drugs, especially oxyphenbutazone, which requires a particular culture environment. Drug activities measured by REMA likely reflect the extracellular activity of the drugs, but the overall effectiveness of a drug is influenced by many factors, including intracellular drug concentrations and activity in environments at varying levels of acidity and varying O2 tensions; therefore any assumptions about the clinical effect derived from MICs are necessarily limited.
Conclusions
By investigating the feasibility of available drugs, MIC findings can help prioritize drugs for further laboratory and clinical research. Our findings provide laboratory data about the bactericidal effect of certain routinely available drugs against MDR M. tuberculosis isolates under extracellular conditions. In particular, trimethoprim/sulfamethoxazole, meropenem/clavulanate, linezolid, clofazimine and nitazoxanide had MICs that were below achievable serum levels or have putative mechanisms of action that suggest enhanced potency in vivo, and all of them are available and have been widely used. Of the many ‘off-the-shelf’ drugs anecdotally reported to have anti-TB activity, clinical investigations of these five drugs should be prioritized.
Acknowledgements
We wish to thank Shiao-Yu Chang and Chao-Chieh Tseng from Taiwan CDC and Lois Diem from US CDC for their excellent technical support.
Other Global PETTS Investigators
The Global Preserving Effective TB Treatment Study Investigators include the following: Joey Lancaster and Ronel Odendaal, Medical Research Council, Pretoria, Republic of South Africa; Lois Diem, Kathrine Tan, Allison Taylor Walker, Erika Sigman and Beverly Metchock, US CDC, Atlanta, GA, USA; M. Therese C. Perez and M. Tarcela Gler, Tropical Disease Foundation, Manila, Philippines; Cesar Bonilla and Oswaldo Jave, Ministry of Health, National TB Strategy, Luis Asencios, NIH, Gloria Yale, Lima City Health District Reference Laboratory, and Carmen Suarez, Lima East Health District Reference Laboratory, Lima, Peru; Inga Norvaisha, Girts Skenders, Ingrida Sture, Vija Riekstina and Andra Cirule, Riga East University Hospital Centre of Tuberculosis and Lung Diseases, Latvia; Sang-Nae Cho, International Tuberculosis Research Center, Changwon, and Yonsei University College of Medicine, Seoul, Republic of Korea; Seokyong Eum and Jongseok Lee, International Tuberculosis Research Center, and Seungkyu Park and Doosoo Jeon, National Masan Hospital, Changwon, Republic of Korea; Ying Cai and Isdore C. Shamputa, NIAID, NIH, Bethesda, MD, USA; Tatiana Kuznetsova, Vladimir Oblast Tuberculosis Dispensary, Russian Federation; Rattanawadee Akksilp, Wanlaya Sitti and Jirapan Inyapong, Office of Disease Prevention and Control Region 7, Ubon Ratchathani, Thailand; Elena V. Kiryanova, Irina Degtyareva and Evgenia S. Nemtsova, Orel Oblast Tuberculosis Dispensary, Russian Federation; Klavdia Levina, North Estonia Regional Hospital, Tallinn; Manfred Danilovits and Tiina Kummik, Tartu University Hospital, Estonia; Yung-Chao Lei and Wei-Lun Huang, Taiwan CDC, Taipei; and Vladislav V. Erokhin, Larisa N. Chernousova, Sofia N. Andreevskaya, Elena E. Larionova and Tatyana G. Smirnova, Central Tuberculosis Research Institute, Russian Academy of Medical Sciences, Moscow.
Funding
Funding for this study was provided by the US Agency for International Development and the US Centers for Disease Control and Prevention (Inter-Agency Agreement: OGH09-011:CGH10-1010196).
Transparency declarations
None to declare.
Disclaimer
The conclusions and interpretations of data presented in this report are solely those of the authors and do not necessarily represent an official position of the US Centers for Disease Control and Prevention or the US government.
References
- 1. Maitra A, Bates S, Kolvekar T. et al. Repurposing—a ray of hope in tackling extensively drug resistance in tuberculosis. Int J Infect Dis 2015; 32: 50–5. [DOI] [PubMed] [Google Scholar]
- 2. Companion Handbook to the WHO Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis. Geneva, Switzerland: WHO, 2015. [PubMed] [Google Scholar]
- 3. Dalton T, Cegielski JP, Akkslip S. et al. Prevalence of and risk factors for resistance to second-line drugs in people with multidrug-resistant tuberculosis in eight countries: a prospective cohort study. Lancet 2012; 380: 1406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palomino JC, Martin A, Camacho M. et al. Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 2002; 46: 2720–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martin A, Palomino JC. 2012. http://tbevidence.org/documents/rescentre/sop/Procedure%20manual%20CRI%2006-2012.pdf.
- 6. Wasserman S, Meintjes G, Maartens G.. Linezolid in the treatment of drug-resistant tuberculosis: the challenge of its narrow therapeutic index. Expert Rev Anti Infect Ther 2016; 14: 901–15. [DOI] [PubMed] [Google Scholar]
- 7. Alsaad N, van Altena R, Pranger AD. et al. Evaluation of co-trimoxazole in the treatment of multidrug-resistant tuberculosis. Eur Respir J 2013; 42: 504–12. [DOI] [PubMed] [Google Scholar]
- 8. Forgacs P, Wengenack NL, Hall L. et al. Tuberculosis and trimethoprim-sulfamethoxazole. Antimicrob Agents Chemother 2009; 53: 4789–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davies Forsman L, Schon T, Simonsson US. et al. Intra- and extracellular activities of trimethoprim-sulfamethoxazole against susceptible and multidrug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother 2014; 58: 7557–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang TS, Kunin CM, Yan BS. et al. Susceptibility of Mycobacterium tuberculosis to sulfamethoxazole, trimethoprim and their combination over a 12 year period in Taiwan. J Antimicrob Chemother 2012; 67: 633–7. [DOI] [PubMed] [Google Scholar]
- 11. Macingwana L, Baker B, Ngwane AH. et al. Sulfamethoxazole enhances the antimycobacterial activity of rifampicin. J Antimicrob Chemother 2012; 67: 2908–11. [DOI] [PubMed] [Google Scholar]
- 12. Ong W, Sievers A, Leslie DE.. Mycobacterium tuberculosis and sulfamethoxazole susceptibility. Antimicrob Agents Chemother 2010; 54: 2748; author reply 2748–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ameen SM, Drancourt M.. In vitro susceptibility of Mycobacterium tuberculosis to trimethoprim and sulfonamides in France. Antimicrob Agents Chemother 2013; 57: 6370–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ogwang S, Good CE, Okware B. et al. Sulfamethoxazole susceptibility of Mycobacterium tuberculosis isolates from HIV-infected Ugandan adults with tuberculosis taking trimethoprim-sulfamethoxazole prophylaxis. Antimicrob Agents Chemother 2015; 59: 5844–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng AC, McBryde ES, Wuthiekanun V. et al. Dosing regimens of cotrimoxazole (trimethoprim-sulfamethoxazole) for melioidosis. Antimicrob Agents Chemother 2009; 53: 4193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alsaad N, van der Laan T, van Altena R. et al. Trimethoprim/sulfamethoxazole susceptibility of Mycobacterium tuberculosis. Int J Antimicrob Agents 2013; 42: 472–4. [DOI] [PubMed] [Google Scholar]
- 17. https://clinicaltrials.gov/ct2/show/NCT01832987.
- 18. Bermudez LE, Meek L.. Mefloquine and its enantiomers are active against Mycobacterium tuberculosis in vitro and in macrophages. Tuberc Res Treat 2014; 2014: 530815.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krieger D, Vesenbeckh S, Schonfeld N. et al. Mefloquine as a potential drug against multidrug-resistant tuberculosis. Eur Respir J 2015; 46: 1503–5. [DOI] [PubMed] [Google Scholar]
- 20. Wernsdorfer WH, Noedl H, Rendi-Wagner P. et al. Gender-specific distribution of mefloquine in the blood following the administration of therapeutic doses. Malar J 2013; 12: 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martins M, Schelz Z, Martins A. et al. In vitro and ex vivo activity of thioridazine derivatives against Mycobacterium tuberculosis. Int J Antimicrob Agents 2007; 29: 338–40. [DOI] [PubMed] [Google Scholar]
- 22. Ordway D, Viveiros M, Leandro C. et al. Clinical concentrations of thioridazine kill intracellular multidrug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother 2003; 47: 917–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thanacoody RH, Daly AK, Reilly JG. et al. Factors affecting drug concentrations and QT interval during thioridazine therapy. Clin Pharmacol Ther 2007; 82: 555–65. [DOI] [PubMed] [Google Scholar]
- 24. Thanacoody HK. Thioridazine: resurrection as an antimicrobial agent? Br J Clin Pharmacol 2007; 64: 566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chang KC, Yew WW, Tam CM. et al. WHO group 5 drugs and difficult multidrug-resistant tuberculosis: a systematic review with cohort analysis and meta-analysis. Antimicrob Agents Chemother 2013; 57: 4097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Diacon AH, Dawson R, von Groote-Bidlingmaier F. et al. Bactericidal activity of pyrazinamide and clofazimine alone and in combinations with pretomanid and bedaquiline. Am J Respir Crit Care Med 2015; 191: 943–53. [DOI] [PubMed] [Google Scholar]
- 27. Schon T, Jureen P, Chryssanthou E. et al. Wild-type distributions of seven oral second-line drugs against Mycobacterium tuberculosis. Int J Tuberc Lung Dis 2011; 15: 502–9. [DOI] [PubMed] [Google Scholar]
- 28. Nix DE, Adam RD, Auclair B. et al. Pharmacokinetics and relative bioavailability of clofazimine in relation to food, orange juice and antacid. Tuberculosis (Edinb) 2004; 84: 365–73. [DOI] [PubMed] [Google Scholar]
- 29. Yawalkar SJ, Vischer W.. Lamprene (clofazimine) in leprosy. Basic information. Lepr Rev 1979; 50: 135–44. [DOI] [PubMed] [Google Scholar]
- 30. Schaad-Lanyi Z, Dieterle W, Dubois JP. et al. Pharmacokinetics of clofazimine in healthy volunteers. Int J Lepr Other Mycobact Dis 1987; 55: 9–15. [PubMed] [Google Scholar]
- 31. Jagannath C, Reddy MV, Kailasam S. et al. Chemotherapeutic activity of clofazimine and its analogues against Mycobacterium tuberculosis. In vitro, intracellular, and in vivo studies. Am J Respir Crit Care Med 1995; 151: 1083–6. [DOI] [PubMed] [Google Scholar]
- 32. Swanson RV, Adamson J, Moodley C. et al. Pharmacokinetics and pharmacodynamics of clofazimine in a mouse model of tuberculosis. Antimicrob Agents Chemother 2015; 59: 3042–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baik J, Rosania GR.. Macrophages sequester clofazimine in an intracellular liquid crystal-like supramolecular organization. PLoS One 2012; 7: e47494.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tyagi S, Ammerman NC, Li SY. et al. Clofazimine shortens the duration of the first-line treatment regimen for experimental chemotherapy of tuberculosis. Proc Natl Acad Sci USA 2015; 112: 869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hwang TJ, Dotsenko S, Jafarov A. et al. Safety and availability of clofazimine in the treatment of multidrug and extensively drug-resistant tuberculosis: analysis of published guidance and meta-analysis of cohort studies. BMJ Open 2014; 4: e004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kurz SG, Bonomo RA.. Reappraising the use of β-lactams to treat tuberculosis. Expert Rev Anti Infect Ther 2012; 10: 999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wivagg CN, Bhattacharyya RP, Hung DT.. Mechanisms of β-lactam killing and resistance in the context of Mycobacterium tuberculosis. J Antibiot 2014; 67: 645–54. [DOI] [PubMed] [Google Scholar]
- 38. Chambers HF, Kocagoz T, Sipit T. et al. Activity of amoxicillin/clavulanate in patients with tuberculosis. Clin Infect Dis 1998; 26: 874–7. [DOI] [PubMed] [Google Scholar]
- 39. Ahmed I, Jabeen K, Inayat R, Hasan R.. Susceptibility testing of extensively drug-resistant and pre-extensively drug-resistant Mycobacterium tuberculosis against levofloxacin, linezolid, and amoxicillin-clavulanate. Antimicrob Agents Chemother 2013; 57: 2522–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dincer I, Ergin A, Kocagoz T.. The vitro efficacy of β-lactam and β-lactamase inhibitors against multidrug resistant clinical strains of Mycobacterium tuberculosis. Int J Antimicrob Agents 2004; 23: 408–11. [DOI] [PubMed] [Google Scholar]
- 41. Solapure S, Dinesh N, Shandil R. et al. In vitro and in vivo efficacy of β-lactams against replicating and slowly growing/nonreplicating Mycobacterium tuberculosis. Antimicrob Agents Chemother 2013; 57: 2506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gonzalo X, Drobniewski F.. Is there a place for β-lactams in the treatment of multidrug-resistant/extensively drug-resistant tuberculosis? Synergy between meropenem and amoxicillin/clavulanate. J Antimicrob Chemother 2013; 68: 366–9. [DOI] [PubMed] [Google Scholar]
- 43. File TM., Jr The development of pharmacokinetically enhanced amoxicillin/clavulanate for the management of respiratory tract infections in adults. Int J Antimicrob Agents 2007; 30 Suppl 2: S131–4. [DOI] [PubMed] [Google Scholar]
- 44. Stewart SM, Fisher M, Young JE. et al. Ampicillin levels in sputum, serum, and saliva. Thorax 1970; 25: 304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carryn S, Chanteux H, Seral C. et al. Intracellular pharmacodynamics of antibiotics. Infect Dis Clin North Am 2003; 17: 615–34. [DOI] [PubMed] [Google Scholar]
- 46. Davies Forsman L, Giske CG, Bruchfeld J. et al. Meropenem-clavulanic acid has high in vitro activity against multidrug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother 2015; 59: 3630–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hugonnet JE, Tremblay LW, Boshoff HI. et al. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science 2009; 323: 1215–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mirza IA, Satti L, Khan FA. et al. In vitro evaluation of linezolid and meropenem against clinical isolates of multi drug resistant tuberculosis by Mycobacterial Growth Indicator Tube (MGIT) 960. J Coll Physicians Surg Pak 2015; 25: 427–30. [PubMed] [Google Scholar]
- 49. De Lorenzo S, Alffenaar JW, Sotgiu G. et al. Efficacy and safety of meropenem-clavulanate added to linezolid-containing regimens in the treatment of MDR-/XDR-TB. Eur Respir J 2013; 41: 1386–92. [DOI] [PubMed] [Google Scholar]
- 50. Palmero D, Gonzalez Montaner P, Cufre M. et al. First series of patients with XDR and pre-XDR TB treated with regimens that included meropenen-clavulanate in Argentina. Arch Bronconeumol 2015; 51: e49–52. [DOI] [PubMed] [Google Scholar]
- 51. Tiberi S, D'Ambrosio L, De Lorenzo S. et al. Ertapenem in the treatment of multidrug-resistant tuberculosis: first clinical experience. Eur Respir J 2016; 47: 333–6. [DOI] [PubMed] [Google Scholar]
- 52. Shigyo K, Ocheretina O, Merveille YM. et al. Efficacy of nitazoxanide against clinical isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother 2013; 57: 2834–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. de Carvalho LP, Lin G, Jiang X. et al. Nitazoxanide kills replicating and nonreplicating Mycobacterium tuberculosis and evades resistance. J Med Chem 2009; 52: 5789–92. [DOI] [PubMed] [Google Scholar]
- 54. Stockis A, Allemon AM, De Bruyn S. et al. Nitazoxanide pharmacokinetics and tolerability in man using single ascending oral doses. Int J Clin Pharmacol Ther 2002; 40: 213–20. [DOI] [PubMed] [Google Scholar]
- 55. Lam KK, Zheng X, Forestieri R. et al. Nitazoxanide stimulates autophagy and inhibits mTORC1 signaling and intracellular proliferation of Mycobacterium tuberculosis. PLoS Pathog 2012; 8: e1002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu H, Wang Y, Liu N. et al. In vitro activities of linezolid against clinical isolates of Mycobacterium tuberculosis from Shenyang, north of China. Indian J Med Microbiol 2015; 33 Suppl: 164–5. [DOI] [PubMed] [Google Scholar]
- 57. Bolhuis MS, van der Laan T, Kosterink JG. et al. In vitro synergy between linezolid and clarithromycin against Mycobacterium tuberculosis. Eur Respir J 2014; 44: 808–11. [DOI] [PubMed] [Google Scholar]
- 58. Yip PC, Kam KM, Lam ET. et al. In vitro activities of PNU-100480 and linezolid against drug-susceptible and drug-resistant Mycobacterium tuberculosis isolates. Int J Antimicrob Agents 2013; 42: 96–7. [DOI] [PubMed] [Google Scholar]
- 59. Pea F, Furlanut M, Cojutti P. et al. Therapeutic drug monitoring of linezolid: a retrospective monocentric analysis. Antimicrob Agents Chemother 2010; 54: 4605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang X, Falagas ME, Vardakas KZ. et al. Systematic review and meta-analysis of the efficacy and safety of therapy with linezolid containing regimens in the treatment of multidrug-resistant and extensively drug-resistant tuberculosis. J Thorac Dis 2015; 7: 603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Koh WJ, Kang YR, Jeon K. et al. Daily 300 mg dose of linezolid for multidrug-resistant and extensively drug-resistant tuberculosis: updated analysis of 51 patients. J Antimicrob Chemother 2012; 67: 1503–7. [DOI] [PubMed] [Google Scholar]
- 62. Gold B, Pingle M, Brickner SJ. et al. Nonsteroidal anti-inflammatory drug sensitizes Mycobacterium tuberculosis to endogenous and exogenous antimicrobials. Proc Natl Acad Sci USA 2012; 109: 16004–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Maitra A, Bates S, Shaik M. et al. Repurposing drugs for treatment of tuberculosis: a role for non-steroidal anti-inflammatory drugs. Br Med Bull 2016; 118: 138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gumbo T. New susceptibility breakpoints for first-line antituberculosis drugs based on antimicrobial pharmacokinetic/pharmacodynamic science and population pharmacokinetic variability. Antimicrob Agents Chemother 2010; 54: 1484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Donald PR, Schaaf HS. Isoniazid pharmacokinetics and efficacy in adults and children In: Donald PR, van Helden PD, eds. Antituberculosis Chemotherapy. Basel, Switzerland: Karger, 2011; 40: 25–31. [Google Scholar]
- 66. Aung KJ, Van Deun A, Declercq E. et al. Successful ′9-month Bangladesh regimen′ for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis 2014; 18: 1180–7. [DOI] [PubMed] [Google Scholar]
- 67. Kuaban C, Noeske J, Rieder HL. et al. High effectiveness of a 12-month regimen for MDR-TB patients in Cameroon. Int J Tuberc Lung Dis 2015; 19: 517–24. [DOI] [PubMed] [Google Scholar]
- 68. Piubello A, Harouna SH, Souleymane MB. et al. High cure rate with standardised short-course multidrug-resistant tuberculosis treatment in Niger: no relapses. Int J Tuberc Lung Dis 2014; 18: 1188–94. [DOI] [PubMed] [Google Scholar]
- 69. Winter HR, Trapnell CB, Slattery JT. et al. The effect of clarithromycin, fluconazole, and rifabutin on sulfamethoxazole hydroxylamine formation in individuals with human immunodeficiency virus infection (AACTG 283). Clin Pharmacol Ther 2004; 76: 313–22. [DOI] [PubMed] [Google Scholar]
- 70. Karbwang J, Na Bangchang K, Thanavibul A. et al. Pharmacokinetics of mefloquine alone or in combination with artesunate. Bull World Health Organ 1994; 72: 83–7. [PMC free article] [PubMed] [Google Scholar]
- 71. Crevoisier C, Handschin J, Barre J. et al. Food increases the bioavailability of mefloquine. Eur J Clin Pharmacol 1997; 53: 135–9. [DOI] [PubMed] [Google Scholar]
- 72. Xu J, Lu Y, Fu L. et al. In vitro and in vivo activity of clofazimine against Mycobacterium tuberculosis persisters. Int J Tuberc Lung Dis 2012; 16: 1119–25. [DOI] [PubMed] [Google Scholar]
- 73. Steel HC, Matlola NM, Anderson R.. Inhibition of potassium transport and growth of mycobacteria exposed to clofazimine and B669 is associated with a calcium-independent increase in microbial phospholipase A2 activity. J Antimicrob Chemother 1999; 44: 209–16. [DOI] [PubMed] [Google Scholar]
- 74. Chakraborty BS, Midha KK, McKay G. et al. Single dose kinetics of thioridazine and its two psychoactive metabolites in healthy humans: a dose proportionality study. J Pharm Sci 1989; 78: 796–801. [DOI] [PubMed] [Google Scholar]
- 75. Stockis A, De Bruyn S, Gengler C. et al. Nitazoxanide pharmacokinetics and tolerability in man during 7 days dosing with 0.5 g and 1 g b.i.d. Int J Clin Pharmacol Ther 2002; 40: 221–7. [DOI] [PubMed] [Google Scholar]
- 76. Li C, Kuti JL, Nightingale CH. et al. Population pharmacokinetic analysis and dosing regimen optimization of meropenem in adult patients. J Clin Pharmacol 2006; 46: 1171–8. [DOI] [PubMed] [Google Scholar]
- 77. Nicolau DP. Pharmacokinetic and pharmacodynamic properties of meropenem. Clin Infect Dis 2008; 47 Suppl 1: S32–40. [DOI] [PubMed] [Google Scholar]