Table 1. Regioselectivity studies using Zn and Al carbenoid reagents a .
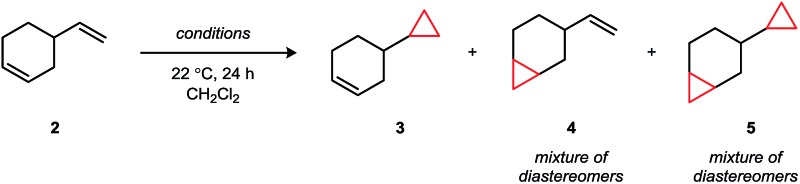
| ||||
| Entry | Reaction conditions | Yield (3 + 4) | rr (3 : 4) | Yield 5 |
| 1 | CH2I2 (1.0 equiv.), Et2Zn (0.5 equiv.) | 28% | 1 : 6.7 | 3% |
| 2 | CH2I2 (1.0 equiv.), Et2Zn (1.0 equiv.) | 33% | 1 : 4.6 | 5% |
| 3 | CH2I2 (2.0 equiv.), Et2Zn (2.0 equiv.) | 53% | 1 : 6.5 | 16% |
| 4 | CH2I2 (1.0 equiv.), Et2Zn (1.0 equiv.), 3,5-difluorobenzoic acid (2.0 equiv.) | 28% | 1 : 3.5 | 19% |
| 5 | CH2I2 (2.0 equiv.), Et2Zn (2.0 equiv.), TiCl4 (0.2 equiv.) | 13% | 1 : 4.6 | 1% |
| 6 | CH2I2 (1.2 equiv.), AlEt3 (1.2 equiv.) | 38% | 1 : 3.1 | 9% |
aReaction conditions: 4-vinylcyclohexene (0.14 mmol), CH2Cl2 (1.0 mL), 24 h, 22 °C. Yields and ratios of regioisomers were determined by GC analysis against an internal standard.
