Table 2. Catalyst structure–activity relationship studies a .
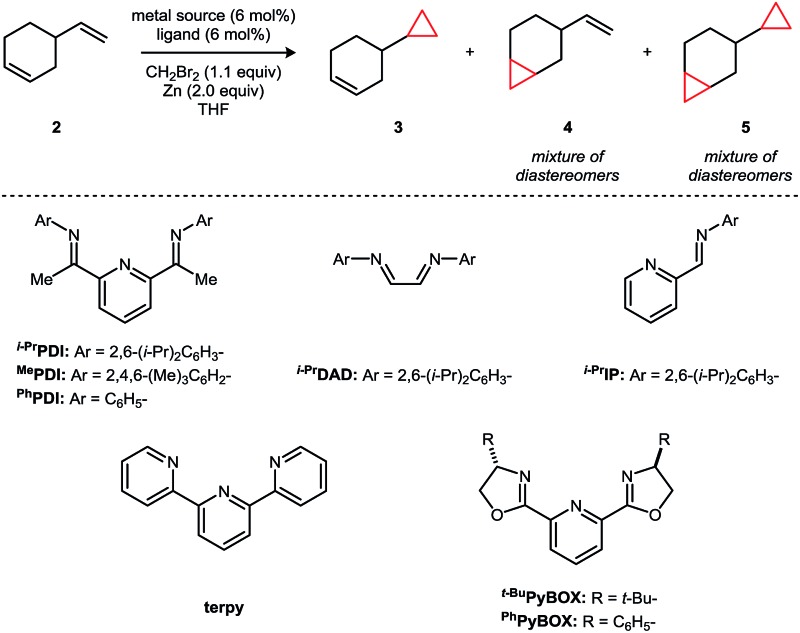
| |||||
| Entry | Metal source | Ligand | Yield (3 + 4) | rr (3 : 4) | Yield 5 |
| 1 | — | — | <1% | — | <1% |
| 2 | CoBr2 | — | <1% | — | <1% |
| 3 | Co(DME)Br2 | — | <1% | — | <1% |
| 4 | — | i–PrPDI | <1% | — | <1% |
| 5 | CoBr2 | i–PrPDI | 81% | >50 : 1 | <1% |
| 6 | CoBr2 | MePDI | 58% | >50 : 1 | <1% |
| 7 | CoBr2 | PhPDI | 4% | — | <1% |
| 8 | CoBr2 | i–PrDAD | <1% | — | <1% |
| 9 | CoBr2 | i–PrIP | 2% | — | <1% |
| 10 | CoBr2 | Terpy | 4% | — | <1% |
| 11 | CoBr2 | t–BuPyBOX | <1% | — | <1% |
| 12 | CoBr2 | PhPyBOX | <1% | — | <1% |
| 13 | CoBr2 | PPh3 (12 mol%) | <1% | — | 0% |
| 14 | FeBr2 | i–PrPDI | 3% | — | <1% |
| 15 | NiBr2 | i–PrPDI | <1% | — | <1% |
aReaction conditions: 4-vinylcyclohexene (0.14 mmol), THF (1.0 mL), 24 h, 22 °C. Yields and ratios of regioisomers were determined by GC analysis against an internal standard.
