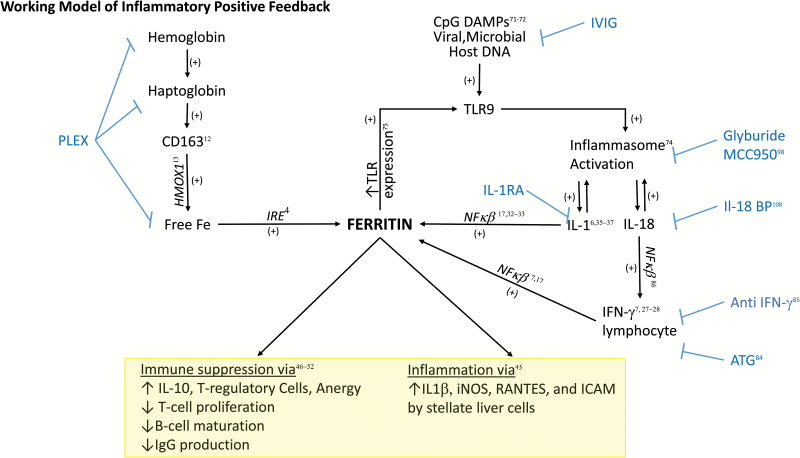
Fig. 4.
Proposed ferritin-mediated feed-forward inflammatory loop. In the setting of ongoing infection, CpG DNA from viral or bacterial pathogens triggers TLR9-mediated signaling. This TLR9 stimulation has been shown to activate inflammasome activity, leading to IL-1β and IL-18 production (71, 74). IL-1β activation can then in turn increase the translation of FTH mRNA through direct interaction with the 5′ UTR (36). Through ferritin’s induction of IL-1β (45) and TLR9 (75), a positive feed back loop would ensue where the generation of ferritin downstream of TLR9 activation would lead to increased amplification of inflammatory signals. Parallel to CpG DAMP-related signaling, infection would be expected to increase the production of free hemoglobin, hemoglobin–haptoglobin complexes (9, 11) and activated CD163+ macrophages, a major cellular producer of ferritin (12, 43). This increased ferritin production could then further amplify the inflammatory loop (12, 43) as well as directly alter lymphocyte function (47–53). Blue text refers to potential therapeutics targeted to steps in the pathway, including using intravenous gamma globulin (IVIG) to clear viral infection and CpG DNA, using plasma exchange (PLEX) for clearance of free hemoglobin and hemoglobin–haptoglobin complexes, as well as free ferritin, using specific anti-IFN-γ antibodies, IL-1RA or IL-18-binding protein (IL-18 BP) or using glyburide to target inflammasome activation.