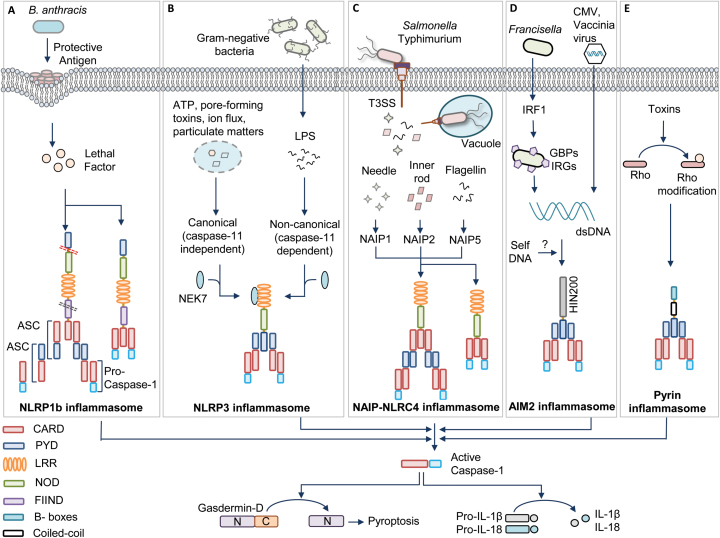
Fig. 1.
Inflammasome activation mechanisms. (A) Bacillus anthracis toxin containing protective antigen and lethal factor activate the human (contains pyrin domain) NLRP1 inflammasome by inducing cleavage at the N-terminal linker region (red dotted lane). Auto-proteolysis at the FIIND domain (black dotted lane) is also required for NLRP1 activation. The NLRP1 inflammasome activates caspase-1 via ASC-dependent recruitment to the inflammasome complex or by direct association with caspase-1 through CARD–CARD interactions. (B) Various pathogen-derived ligands (PAMPs) and physiological aberrations (DAMPs) activate NLRP3. Assembly of the NLRP3 inflammasome is categorized into canonical (caspase-11 independent) and non-canonical (caspase-11 dependent) inflammasome activation. NEK7 is an upstream activator of NLRP3 inflammasome assembly. (C) Pathogenic bacteria such as Salmonella enterica subspecies Typhimurium operate a T3SS to release effector proteins into the cytosol. These pathogen-associated proteins are recognized by a family of NAIPs and they further recruit NLRC4 to assemble the inflammasome complex. NLRC4 enables ASC-dependent or direct CARD–CARD interaction-dependent casapse-1 activation. (D) The DNA viruses such as cytomegalovirus (CMV) and vaccinia virus and intracellular bacteria such as Francisella release DNA during infection for activating the AIM2 inflammasome. IRF1 induces the expression of GBPs and IRGs to liberate DNA for AIM2 recognition. (E) Pyrin detects the modifications of Rho induced by Rho-inactivating toxins. All these inflammasomes recruit an adaptor protein called ASC, which contains PYD and CARD. ASC further brings caspase-1 to the inflammasome complex by CARD–CARD interactions for its activation. Activated caspase-1 drives the cleavage of pro-inflammatory cytokines pro-IL-1β and pro-IL-18 and also the processing of gasdermin-D protein for executing pyroptosis.