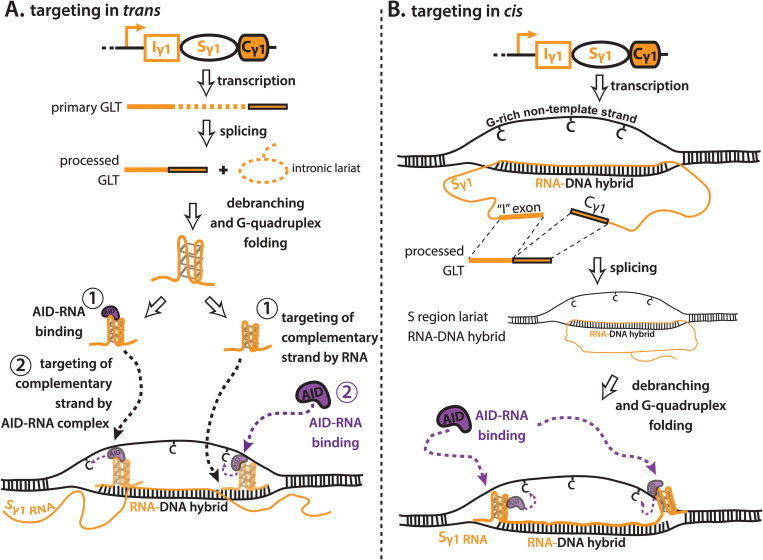
Fig. 5.
Model for RNA-dependent targeting of AID during class switch recombination. (A) Targeting of AID–RNA complexes in trans. Germline transcripts (GLTs) are transcribed from downstream CH regions upon B cell activation, and undergo splicing to form a processed GLT and an intronic switch (S) region lariat. The S region lariat is debranched to form a linear S region transcript, which can fold into a G-quadruplex secondary RNA structure endowed by the G-richness of S region non-template strand DNA. It is currently unknown if debranching is required for G-quadruplex folding. G-quadruplexes can interact with soluble AID, and AID–RNA complexes are guided to the S region by the strand complementarity between the G-quadruplex and the S region from which it was transcribed (left path). Alternatively, the G-quadruplex can target to the S region independently of AID binding, and then recruit AID via AID–RNA binding (right path). For trans targeting, G-quadruplexes must compete with existing RNA–DNA hybrids for access to template-strand DNA. (B) Targeting of AID–RNA complexes in cis. GLTs are produced from downstream CH regions upon B cell activation; however, they remain attached to their template strand DNA via formation of an R-loop. The GLT undergoes splicing to liberate a processed GLT, while the S region lariat remains annealed as part of the R-loop. Debranching and folding into G-quadruplex secondary RNA structures mediates the recruitment of AID via AID–RNA binding. For cis targeting, G-quadruplexes are not required to compete with RNA–DNA hybrids for access to template-strand DNA; AID, activation-induced cytidine deaminase.