Figure 14.
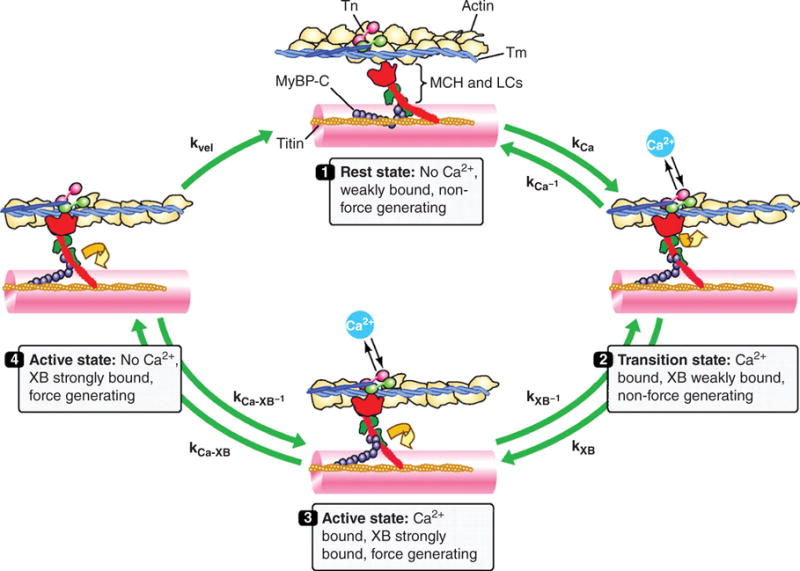
Schematic drawing of the cardiac cross-bridge cycle. Thin-filaments are shown with actin, tropomyosin (Tm) and the troponin (Tn) complex with the Ca2+-binding unit (cTnC) in pink, the Tm-binding unit (cTnT) in blue, and the inhibitory unit (cTnI) in light green. Thick-filament cross-bridges (XB) are shown with myosin heavy chain (MHC; figure illustrating one MHC) in red, myosin light chains (LC) in green, along with myosin-binding protein C (MyBP-C) in purple and titin in orange. Cross-bridges are initially in a rest state (1) where they are weakly bound and do not generate force. Cross-bridges enter a transition state (2) determined by the on (kCa) and off rates (kCa-1) for Ca2+ exchange with cTnC. During this transition state, cross-bridges are weakly bound (kXB-1) and do not generate force. In the active state (3), the cTnT-dependent shift of Tm from its blocking position on actin filaments allows strong cross-bridge binding (kXB) and induces cooperative activation of the thin filament (e.g., increase Ca2+ affinity of cTnC; kCa-XB-1). In the active state (4) with loss of bound Ca2+, the cooperative mechanisms allow a population of cross-bridges to remain active and force generating (kCa-XB). Mechanical feedback termed shortening-induced deactivation (kvel) will transition active cross-bridges back to the resting state. [Fig. modified, with permission, from (265).]
