Figure 8.
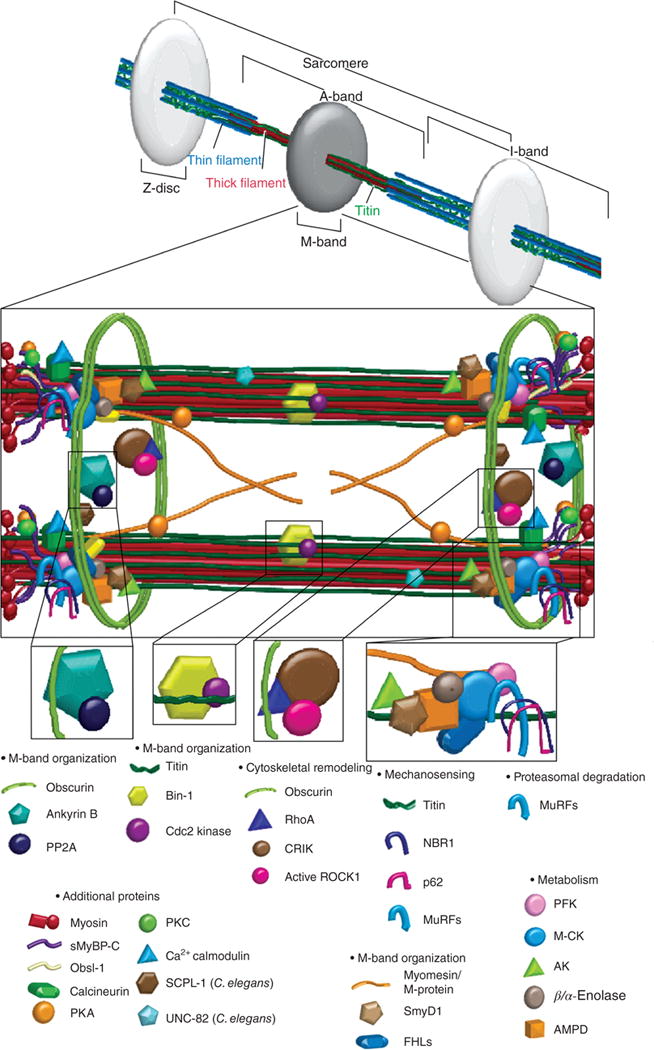
The sarcomeric M-band contains components important for mechanosensing, proteosomal degradation, actin dynamics, metabolism, and signal transduction. Myomesin is a key structural protein of the M-band. MURFs (muscle-specific ring finger protein) are multifunctional proteins that ubiquitinate certain myofibrillar proteins, play a key role in muscle atrophy and regulate hypertrophic signaling. Obscurin interacts with ankyrin and anchors the sarcomere to the sarcoplasmic reticulum; ankyrin and obscurin also sequester PP2A (protein phosphatase 2A) to the M-band. FHLs (four-and-a-half LIM proteins) bind to titin’s N2B spring region and activate downstream signaling pathways, thus serving as an important mechanosensor that triggers hypertrophy in response to strain. FHL2 also docks important metabolic enzymes such as the metabolic enzymes muscle-specific M-CK (creatine kinase), AK (adenylate kinase), and PFK (phosphofructokinase). M-CK anchors the glycolytic enzyme β/α-enolase to the M-band. The muscle isoform of AMPD (adenosine monophosphate deaminase) works with M-CK and AK to monitor local ATP levels. Other proteins identified at the M-band, but not discussed in this review include SmyD1, SCPL-1 (Caenorhabditis elegans), UNC-82 (C. elegans), p62, rhoA, CRIK, and active ROCK1. [Fig. reprinted, with permission, from (277).]
