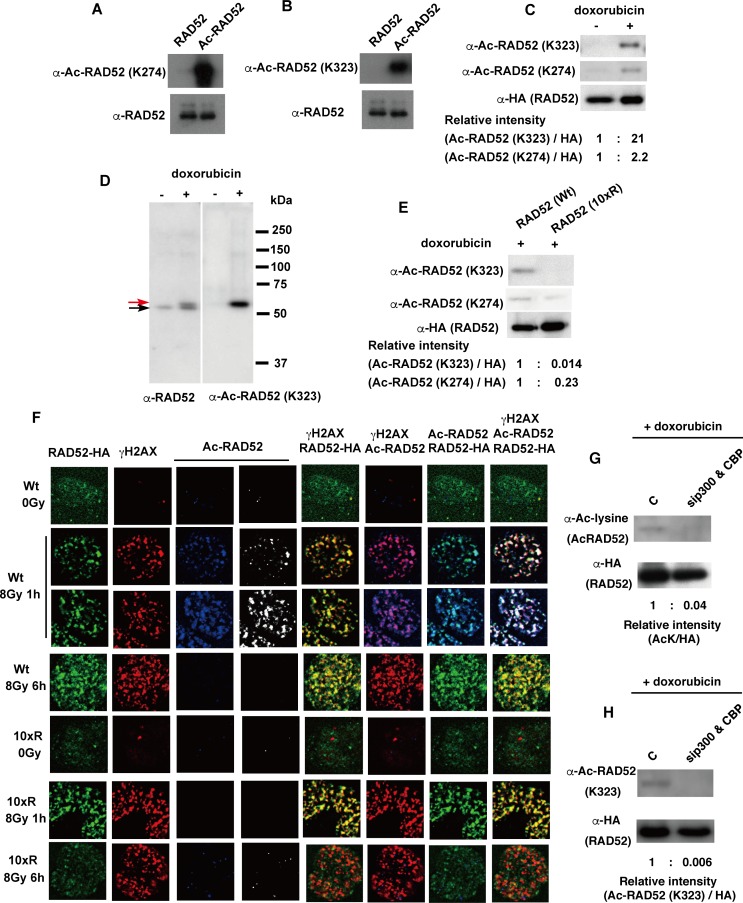
Fig 3. Human RAD52 is acetylated in vivo.
(A, B) An in vitro acetylation assay was performed by incubating RAD52 (0.25 μg) in 10 μl HAT buffer A containing 10 mM sodium butyrate, in the presence of 0.5 μg Ac-CoA and CBP-FLAG (54 ng), at 30°C for 60 min. Unacetylated and acetylated RAD52 were subjected to immunoblotting analyses using the indicated antibodies. (C, D, E) Acetylation of the FLAG-RAD52-HA protein purified from MSCs was detected as described in the Supporting Materials and Methods, using the indicated antibodies. The expression plasmid, pT-Rex-DEST30 containing FLAG-RAD52 (Wt)-HA or FLAG-RAD52 (10xR)-HA, was transfected into the cells. FLAG-RAD52-HA was purified from cell extracts 24 h after transfection. Cells were treated with doxorubicin for 2 h, as indicated. (D) The red arrow indicates the mobility-shifted band. (F) MSCs expressing FLAG-RAD52 (Wt)-HA or FLAG-RAD52 (10xR)-HA were unirradiated or irradiated with γ-rays (8 Gy). At the indicated time after irradiation, the cells were subjected to immunofluorescent staining with anti-HA (green), anti-γH2AX (red), and anti-acetylated RAD52 at K323 (blue or white) antibodies. (G, H) T-Rex-293 cells expressing FLAG-RAD52-HA were transfected with either a negative control siRNA or mixture of p300 and CBP-specific siRNAs. At 24 h after transfection, the cells were treated with doxorubicin for 2 h. Immunoprecipitated FLAG-RAD52-HA proteins from the cell extracts were subjected to immunoblotting analyses with the indicated antibodies. (C, E, G, H) The relative band intensities normalized to those of the HA bands are shown below the immunoblots.