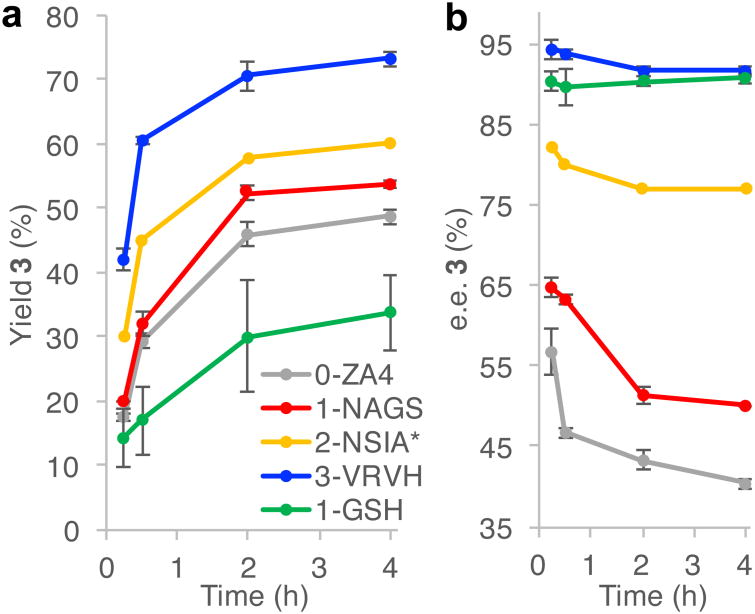
Figure 6. Time course experiments of ArM catalyzed cyclopropanations of styrene with (4-methoxyphenyl) methyldiazoacetate.
(see Supplementary Table 2 for full data). a, Plot of cyclopropane yield versus time, showing increases for each generation of mutants. b, Plot of cyclopropane enantiomeric excess (e.e.) versus time. A decrease in %e.e. over the course of reaction was observed, which decreases along the lineage. The cause of this was investigated but not identified (see Main Text). Reactions were performed in triplicate with standard deviation shown. *2-NSIA reactions were performed in duplicate, prohibiting standard deviation calculation.