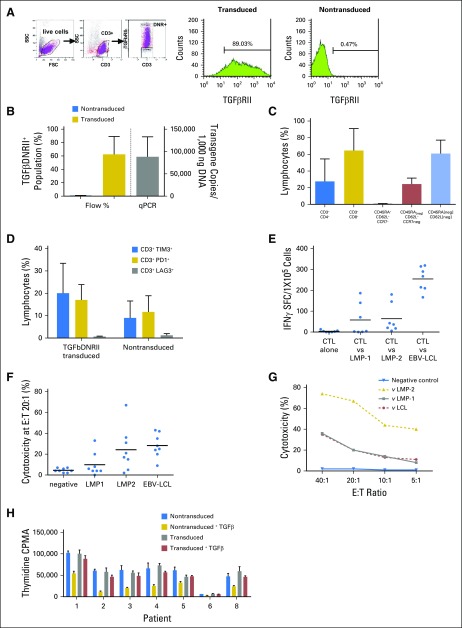
Fig 1.
Characteristics of infused dominant-negative transforming growth factor-β (TGF-β) type 2 receptor (DNRII) latent membrane protein (LMP)–specific T cells (DNRII-LSTs). (A) Gating strategy for detecting DNRII-transduced cells by flow cytometry. T cells that were transduced with SFG:DNRII (truncated TGFβRII) were stained with anti-CD3 and TGFβRII monoclonal antibody. Expression of the transgenic TGFβRII receptor was determined on the basis of comparison with nontransduced LSTs. (B) DNRII expression is shown as both percentage of TGFβDNRII expression on T cells by flow cytometry (blue bar represents nontransduced cells and gold bar represents transduced cells; n = 5) and the number of copies on quantitative PCR (qPCR; gray bar represents transduced cells; n = 8). A mean of 62.44% (range, 23.27% to 89.03%) of CD3+ T cells transduced with the clinical-grade SFG:DNRII vector expressed the mutant receptor construct (DNRII) as determined by flow cytometry. Transgene levels ranged from 35,841 to 168,408 transgene copies/100 ng DNA. (C) Phenotypic characteristics of DNRII-LSTs as determined by flow cytometry. Percentages were based on the lymphocyte gate and are expressed as means ± standard deviation (n = 8). DNRII-LSTs displayed a predominantly effector memory phenotype (CD45RAneg/CCR7neg/CD62Lneg) (mean, 61%; range, 40% to 76%) with populations demonstrating a CD45RAneg/CCR7neg/CD62Lpos phenotype that was intermediate between central memory (CCR7+CD45RA−) and effector memory (mean, 31.03%; range, 12.17% to 56.56%). (D) Expression of the exhaustion markers, TIM3, PD1, and LAG3, as determined by flow cytometry. Percentages were based on the lymphocyte gate and are expressed as means ± standard deviation (n = 4). The exhaustion markers, TIM3 and PD1, were expressed on a mean of 20% (range, 2.4% to 33.3%) and 17% (range, 9.7% to 22.9%), respectively, of transduced cells compared with means of 9% (range, 0.4% to 18.6% TIM3) and 11.7% (range, 7.7% to 22.5% programmed death-1 [PD1]) in nontransduced T-cell products. However, LAG3 was negative in all products. (E) Ex vivo expanded DNRII-LSTs are specific for Epstein-Barr virus (EBV) antigens, LMP-1 and LMP-2, and recognize EBV antigen–expressing autologous lymphoblastoid cell lines (EBV-LCL), as measured by interferon-gamma (IFN-γ) enzyme-linked immunospot (ELISPOT). Black lines indicate mean values (n = 7). Transduced T cells demonstrated LMP specificity as evaluated by IFNγ ELISPOT assay (median, 4.5 spot-forming cells/1 × 105cells; range, 0 to 186.5 for LMP-1; median, 36.5 spot-forming cells/1 × 105cells; range, 6 to 146 for LMP-2). (F) DNRII-LSTs lyse EBV antigen–expressing autologous targets as measured by chromium release assay. Specific lysis at 20:1 effector:target ratio (E:T; except for patient 7, denoted as a star, at 40:1) is shown. Black lines indicate mean values (n = 8). (G) Representative plot of cytotoxicity as measured by chromium release assay at different E:T ratios (patient 8). (H) Proliferation assay that compare thymidine uptake in nontransduced (gold bars) and transduced cells (red bars) in the presence and absence (blue and gray bars, respectively) of transforming growth factor-β. Bars show means ± standard deviation of thymidine uptake counts per minute (CPMA; n = 7). FSC, forward scatter; SFC, spot-forming cell; SSC, side scatter.