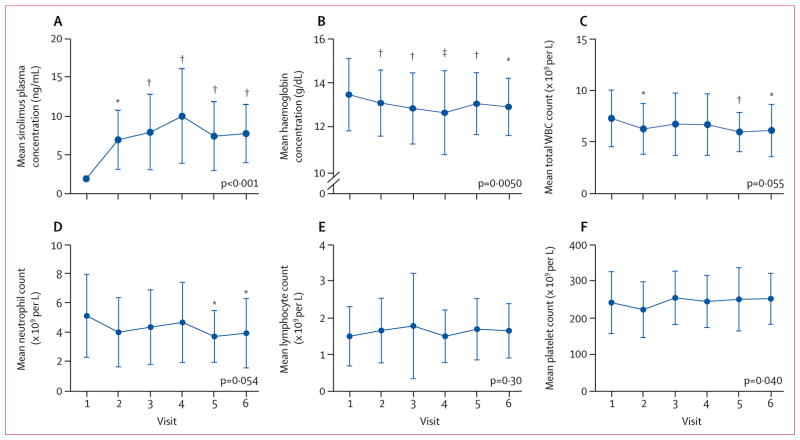
Figure 1. Safety outcomes.
Mean sirolimus plasma concentration (A), mean haemoglobin concentration (B), mean total WBC count (C), and mean neutrophil (D), lymphocyte (E), and platelet counts (F) were measured before treatment (visit 1) and after initiation of treatment at 1 month (visit 2), 3 months (visit 3), 6 months (visit 4), 9 months (visit 5), and 12 months (visit 6) in 40 patients with systemic lupus erythmatosus. Overall changes in safety endpoints during treatment were assessed by repeated measures analysis using a mixed-effects model logistic regression approach with exact p values indicated for each safety outcome. Changes in safety endpoints at each visit (visits 2–6) were assessed by two-tailed paired t tests relative to visit 1. Error bars show SD. WBC=white blood cell. *p<0·05. †p<0·01. ‡p<0·001.