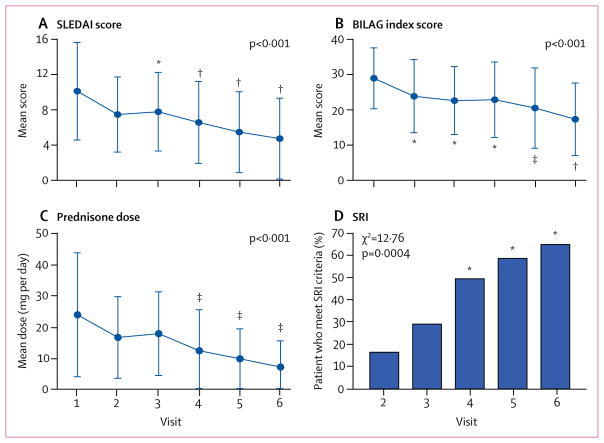
Figure 2. Clinical efficacy outcomes.
Mean SLEDAI score (A), BILAG index score (B), and daily prednisone dose (C) at baseline (visit 1) and during treatment (visits 2–6). Overall changes in SLEDAI, BILAG, and prednisone dosage during sirolimus treatment were assessed by repeated measures analysis using a mixed model logistic regression approach with exact p values indicated for each outcome. Changes in SLEDAI, BILAG, and prednisone dosage at each visit (visits 2–6) were also assessed by two-tailed paired t test relative to visit 1. (D) The proportion of patients who met responder criteria according to the SRI at visits 3–6 were compared with that at visit 2 using visit 1 as a reference point. Overall distribution of responders and non-responders for SRI at visits 2–6 were also assessed by two-tailed χ2 test. Error bars show SD. SLEDAI=Systemic Lupus Erythematosus Disease Activity Index. BILAG=British Isles Lupus Assessment Group. SRI=Systemic Lupus Erythematosus Responder Index. *p<0·01. †p<0·001. ‡p<0·05.