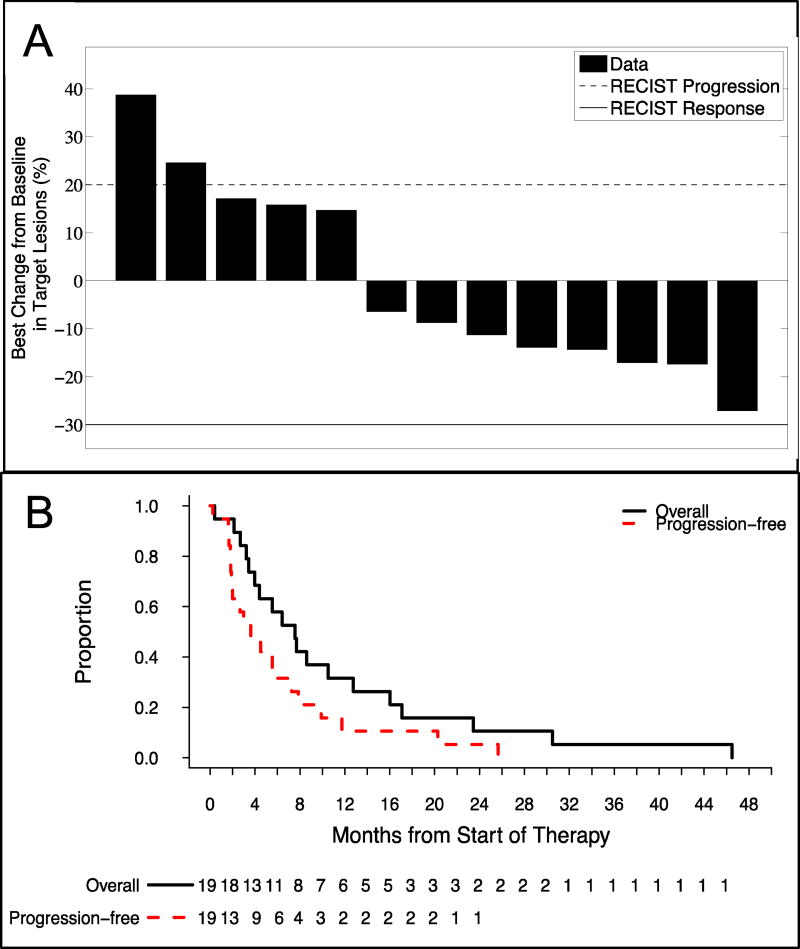
Figure 2. Antitumor Activity.
(A) Best percentage change from baseline in the sum of all target lesions is presented for each efficacy evaluable patient on study. Solid and dashed lines represent the RECIST v1.1 definition for partial response and progressive disease, respectively. Although no responses were observed, eight (62%) of patients had reductions in tumor size suggesting the triplet combination has some antitumor activity in patients with advanced pancreatic carcinoma. (B) Kaplan-Meier analysis of progression-free survival (dashed red line) and overall survival (block solid line). The overall median progression-free survival was 3.6 months whereas the median overall survival was 8.0 months.