Table 3.
Anti-angiogenesis properties of substituted phthalimidines.
| compound | structure | % nitritea | vessel lengthb | # of vesselsc |
|---|---|---|---|---|
| 11 |
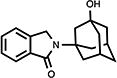
|
101 | 105.1±4.9 | 19.5±0.5 |
| 21 |
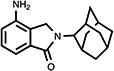
|
72 | 42.1±3.3 | 14.0±1.4 |
| 9 |
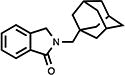
|
50 | 83.1±2.4 | 18.2±0.4 |
| 22 |
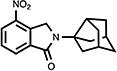
|
49 | 42.8±9.2 | 8.6±2.7 |
| 24 |
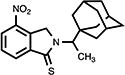
|
11.5 | 86.4±2.8 | 22.0±0.5 |
| 20 |
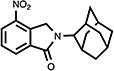
|
9.2 | 48.2±1.9d | 14.6±0.6d |
nitrite data extrapolated from Table 1; presented as mean % of control
vessel length in µm, obtained at 10µg/mL; presented as mean ± S.E.M
vessel number obtained at 10µg/mL; values presented as mean ± S.E.M
compound 20 screened at 5µg/mL; values presented as mean ± S.E.M
