Abstract
Background
The phase III Japanese Rivaroxaban Once‐Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (J‐ROCKET AF) showed that the rivaroxaban group had a lower event rate of intracranial bleeding than the warfarin group and that rivaroxaban was noninferior to warfarin for the principal safety outcome. However, safety and effectiveness data from unselected patients with AF in everyday clinical practice in Japan are lacking.
Methods
The Xarelto Post‐Authorization Safety & Effectiveness Study in Japanese Patients with Atrial Fibrillation (XAPASS) is a real‐world, prospective, single‐arm, observational study mandated by the Japanese authority as postmarketing surveillance. XAPASS involves patients with nonvalvular AF prescribed rivaroxaban. The principal safety outcome is a composite of major and nonmajor bleeding events, and the primary effectiveness outcome is the incidence of ischemic stroke, hemorrhagic stroke, noncentral nervous system systemic embolism, and myocardial infarction.
Results
In total, 11 308 patients were enrolled from April 2012 to June 2014. Their age was 73.1 ± 9.9 years, and their CHADS 2 score was 2.2 ± 1.3. Female patients, patients aged ≥75 years, patients with a body weight of ≤50 kg, and patients with a creatinine clearance of <50 mL/min constituted 38.1%, 48.7%, 19.5%, and 23.9% of all patients, respectively. Almost half (53.2%) of patients were prescribed other anticoagulants before starting rivaroxaban.
Conclusions
Data from this study will supplement those from the J‐ROCKET AF and provide practical information for the optimal use of rivaroxaban for stroke prevention in Japanese patients with AF (Clinicaltrials.gov: NCT01582737).
Keywords: anticoagulants, atrial fibrillation, postmarketing surveillance, rivaroxaban, stroke prevention
1. INTRODUCTION
Atrial fibrillation (AF) is the most common sustained arrhythmia, and its prevalence of approximately 1% in the Japanese population is steadily increasing due to the country's aging population.1, 2, 3 Without anticoagulation, AF is associated with a fourfold to fivefold increase in the risk of stroke.4, 5 Although oral anticoagulants (OACs) effectively reduce the risk of stroke in patients with AF, warfarin (the only OAC available in Japan before 2011) has been underused in real‐world clinical practice because of significant clinical limitations such as drug and food interactions and the need for frequent coagulation monitoring. Since 2011, nonvitamin K antagonist OACs (NOACs) including dabigatran, rivaroxaban, apixaban, and edoxaban have been approved in Japan for stroke prevention in patients with nonvalvular AF (NVAF) and are now widely used in clinical practice as recommended by the guidelines.6
One of these NOACs, rivaroxaban (BAY59‐7939), is a novel, oral, direct factor Xa inhibitor that inhibits thrombin formation via a different mechanism of action than that of warfarin. Rivaroxaban offers benefits over warfarin such as a rapid onset of action, no requirement to conduct monitoring for dose adjustment, and fewer interactions with food and concomitant drugs.7 In 2012, rivaroxaban received regulatory approval in Japan for stroke prevention in patients with NVAF based on the results of the phase III Japanese Rivaroxaban Once‐Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (J‐ROCKET AF; NCT00494871)8 and Rivaroxaban Once‐Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF; NCT00403767).9 The ROCKET AF evaluated the safety and efficacy of rivaroxaban 20 mg once daily (od) (15 mg od in patients with moderate renal impairment, defined as a baseline creatinine clearance [CrCl] of 30‐49 mL/min) for the prevention of stroke and systemic embolism (SE) in patients with NVAF. In contrast, 15 mg od (10 mg od in patients with moderate renal impairment) was selected for phase III evaluation in the J‐ROCKET AF, according to differences in drug exposure between Japanese and Caucasian patients and lower international normalized ratio targets in Japanese clinical practice as recommended by Japanese guidelines.
In the phase III J‐ROCKET AF, rivaroxaban was compared with dose‐adjusted warfarin for the prevention of stroke and SE in high‐risk Japanese patients with NVAF. Rivaroxaban was noninferior to warfarin for the principal safety outcome (hazard ratio [HR], 1.11; 95% confidence interval [CI], 0.87‐1.42; P noninferiority < .001), and the rivaroxaban group had a lower event rate of intracranial bleeding than the warfarin group (0.8% vs 1.6%, respectively). There was a strong trend for a lower rate of stroke/SE with rivaroxaban than warfarin (HR, 0.49; 95% CI, 0.24‐1.00; P = .050).
Postauthorization studies are needed to fully reveal the safety and effectiveness of new agents in routine clinical practice. Because of their strict design requirements, such as well‐defined inclusion and exclusion criteria, phase III clinical trials may not fully reflect the characteristics observed in the broad range of patients seen in routine clinical practice. Therefore, this postmarketing surveillance registry was planned to explore the safety and effectiveness of rivaroxaban in patients with NVAF in real‐world clinical practice in Japan. This article describes and discusses the study design and baseline characteristics of the enrolled patients.
2. METHODS
This postmarketing surveillance study was approved by the Ministry of Health, Labour, and Welfare (MHLW) and will be carried out in accordance with the standards for Good Post‐marketing Study Practice provided by the MHLW in Japan.
2.1. Objectives
The Xarelto Post‐Authorization Safety & Effectiveness Study in Japanese Patients with Atrial Fibrillation (XAPASS; NCT01582737) is a real‐world, prospective, postauthorization, observational study mandated by a Japanese regulatory authority, namely the Pharmaceuticals and Medical Devices Agency, as postmarketing surveillance. In contrast to the phase III J‐ROCKET AF, the XAPASS procedures do not interfere with the clinical management of patients with NVAF or with the prescribing behaviors of attending physicians because study is designed to assess the use of rivaroxaban in real‐world clinical practice. The key goal of the XAPASS is to confirm the safety profile of rivaroxaban in real‐world use in Japan across a broad range of patients with NVAF.
2.2. Study design
Xarelto Post‐Authorization Safety & Effectiveness Study in Japanese Patients with Atrial Fibrillation is an open‐label, single‐arm, observational, noninterventional cohort study (Figure 1). The standard observation period for each patient is 2 years; data are collected 6 months, 1 year, and 2 years after the initiation of rivaroxaban treatment. After the completion of the standard observation period, follow‐up investigations are being conducted for a maximum of 5 years.
Figure 1.
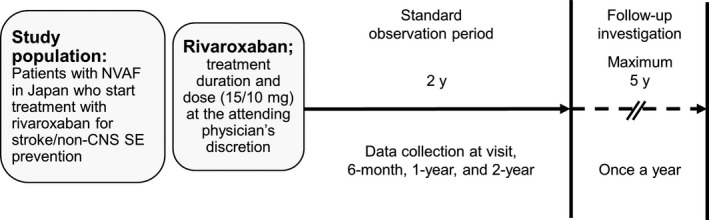
The XAPASS single‐arm, prospective, observational design. Non‐CNS SE, noncentral nervous system systemic embolism; NVAF, nonvalvular atrial fibrillation
2.3. Patient population
Eligible patients comprise men or women with NVAF starting rivaroxaban therapy to reduce the risk of stroke/SE. Contraindications to rivaroxaban therapy were considered according to the Japanese package insert.
2.4. Drug administration
In Japan, rivaroxaban at 15 and 10 mg od is approved for patients with a CrCl of ≥50 and <50 mL/min, respectively. Treating physicians prescribe rivaroxaban at their discretion, including the dose (15 or 10 mg od) and duration of therapy. Any use of an anticoagulant or antiplatelet agent ≤30 days prior to rivaroxaban administration is documented in case report forms (CRFs), alongside details of any medications or other therapies. The reasons for discontinuation of rivaroxaban treatment and any follow‐on therapy are documented in the CRF; any temporary interruptions of rivaroxaban therapy are also documented.
2.5. Baseline data
The baseline data collected were as follows.
Age, sex, body weight, height, smoking history, and any history of allergy
History of NVAF including date of onset and type (paroxysmal, persistent, or permanent)
Use of an anticoagulant or antiplatelet agent ≤30 days prior to rivaroxaban administration
Other medical history
Vital signs and laboratory tests, if performed as part of routine care
CrCl (mL/min)
Stroke and bleeding risk profiles based on risk scores such as CHADS2 (congestive heart failure, hypertension, age, diabetes mellitus, stroke), CHA2DS2‐VASc (congestive heart failure, hypertension, age of ≥75 years, diabetes mellitus, stroke, vascular disease, age of 65‐74 years, sex category), or HAS‐BLED (hypertension, abnormal liver/renal function, stroke history, bleeding predisposition, labile international normalized ratio, elderly, drug/alcohol use)
Child‐Pugh score
2.6. Study outcomes
The primary outcomes are those that allow for assessment and estimation of the safety of rivaroxaban in routine clinical practice, particularly in patients weighing ≤50 kg and those aged ≥75 years. These outcomes will be recorded as adverse events (AEs) or serious AEs, which will comprise bleeding events (major and nonmajor bleeding events, principal safety outcome) and effectiveness events (stroke [ischemic or hemorrhagic], noncentral nervous system SE, or myocardial infarction, primary effectiveness outcome). Major bleeding is defined as clinically overt bleeding associated with any of the following: fatal outcome, involvement of a critical anatomic site (intracranial, spinal, ocular, pericardial, articular, retroperitoneal, or intramuscular with compartment syndrome), >2‐g/dL reduction in hemoglobin concentration, transfusion of >2 units of whole blood or packed red blood cells, or permanent disability. Nonmajor bleeding is defined as overt bleeding not meeting the criteria for major bleeding. Stroke is defined as a new sudden, focal neurological deficit resulting from a presumed cerebrovascular cause, persisting beyond 24 hours and unattributable to another readily identifiable cause. Noncentral nervous system SE is defined as abrupt vascular insufficiency associated with clinical or radiological evidence of arterial occlusion in the absence of other likely mechanisms (eg, trauma, atherosclerosis, or instrumentation). Myocardial infarction is defined as typical symptoms plus elevation in the levels of cardiac biomarkers (troponin I, troponin T, or creatinine kinase‐MB) above the upper limit of normal, new pathological Q waves in ≥2 contiguous electrocardiographic leads, or confirmation at autopsy.
Secondary outcomes include all‐cause mortality, treatment persistence with rivaroxaban, and rates of AEs or serious AEs across patients with different baseline risk profiles for stroke or bleeding (eg, CHADS2, CHA2DS2‐VASc, or HAS‐BLED), other baseline subgroups (eg, age, body weight, CrCl, use of antiplatelet agents, or prior stroke/transient ischemic attack/noncentral nervous system SE), and doses (15 or 10 mg).
2.7. Statistical analysis plan
Statistical analyses are descriptive, exploratory, and generally limited to frequency tables or summary statistics (eg, mean ± standard deviation or median ± quartile for continuous variables and frequency or percentage for categorical variables), for example, for demographic data. Events of interest are presented as both raw incidence proportions (patients with events/number of treated patients) and incidence rates (eg, patients with events per 100 patient‐years). Each estimate is presented with the corresponding 95% CI. Kaplan‐Meier plots will show the time course up to the first event of interest. Multivariate data analysis is also planned.
2.8. Data management
Data from the XAPASS are captured in electronic CRFs, which comprise three parts. The investigating physician enters and transmits the information for all targeted patients already enrolled as follows: CRF 1 records patient background characteristics and the observation status for months 1‐6, CRF 2 records the observation status for months 7‐12, and CRF 3 records the observation status for months 13‐24. During the 5‐year follow‐up period, information is entered and transmitted every 1 year after termination of the standard observation period. If rivaroxaban therapy is discontinued, the observation period continues for a further 30 days. The XAPASS uses one centralized database to receive results, and data are analyzed by an independent data center. The data as of September 2017 were used for this study.
2.9. Administrative organization
The XAPASS is a postmarketing surveillance study funded by Bayer Yakuhin Ltd. (Osaka, Japan) and conducted under the supervision of a steering committee (Appendix A) that developed the protocol and provides oversight of study execution, oversees the database, and is accountable for analysis of the results and publications. Operational oversight of the study will be performed through collaboration between the steering committee and Bayer Yakuhin Ltd.
3. RESULTS
3.1. Baseline characteristics
In total, 11 308 Japanese patients with NVAF prescribed rivaroxaban were enrolled from 1416 institutions from April 2012 to June 2014 (the date of the first patient's first visit was 18 April 2012). Outpatients constituted 84.6% of patients, while inpatients constituted 15.4%. Baseline characteristics are summarized in Table 1. The age was 73.1 ± 9.9 years, and 48.7% of patients were aged ≥75 years. Female patients constituted 38.1%. The body weight was 60.9 ± 12.6 kg, and 19.5% of patients had a body weight of ≤50 kg. The body mass index was 23.7 ± 3.8 kg/m2. The CrCl was 67.7 ± 28.9 mL/min, and 23.9% of patients had a CrCl of <50 mL/min. The CHADS2 score was 2.2 ± 1.3. Patients with hypertension, diabetes mellitus, previous stroke/transient ischemic attack, and congestive heart failure constituted 74.3%, 22.3%, 23.7%, and 25.0% of all patients, respectively. Among 6017 (53.2%) patients treated with other anticoagulants prior to the administration of rivaroxaban, 3960 (65.8%), 1688 (28.1%), and 369 (6.1%) were treated with warfarin, dabigatran, and other anticoagulants, respectively. Figure 2 shows the histograms of age, body weight, CrCl, and CHADS2 score. Table 1 also shows the baseline characteristics of 5396 (47.7%) patients from clinics with ≤19 beds and 5912 (52.3%) patients from hospitals with ≥20 beds. Patient characteristics according to geographic region are shown in Table S1.
Table 1.
Baseline characteristics
| Characteristic | All patients (N = 11 308) | Patients from clinics with beds ≤19 (N = 5396) | Patients from hospitals with beds ≥20 (N = 5912) |
|---|---|---|---|
| Age‐y | 73.1 ± 9.9 | 73.2 ± 9.8 | 73.1 ± 9.9 |
| <75 y‐no. (%) | 5804 (51.3) | 2764 (51.2) | 3040 (51.4) |
| ≥75 y‐no. (%) | 5504 (48.7) | 2632 (48.8) | 2872 (48.6) |
| Female sex‐no. (%) | 4306 (38.1) | 2125 (39.4) | 2181 (36.9) |
| Height‐cm | 160.2 ± 9.9 | 160.0 ± 10.1 | 160.3 ± 9.7 |
| Body weight‐kg | 60.9 ± 12.6 | 61.2 ± 12.6 | 60.7 ± 12.7 |
| Body weight‐no. (%) | |||
| ≤50 kg | 2209 (19.5) | 1011 (18.7) | 1198 (20.3) |
| >50 kg | 8081 (71.5) | 3887 (72.0) | 4194 (70.9) |
| Unknown | 1018 (9.0) | 498 (9.2) | 520 (8.8) |
| BMI‐kg/m2 | 23.7 ± 3.8 | 23.9 ± 3.6 | 23.6 ± 3.9 |
| BMI‐no. (%) | |||
| <18.5 | 615 (5.4) | 228 (4.2) | 387 (6.6) |
| 18.5 to <25 | 5348 (47.3) | 2495 (46.2) | 2853 (48.3) |
| 25 to <30 | 2493 (22.0) | 1230 (22.8) | 1263 (21.4) |
| ≥30 | 483 (4.3) | 232 (4.3) | 251 (4.3) |
| Unknown | 2369 (20.9) | 1211 (22.4) | 1158 (19.6) |
| Creatinine clearance‐mL/min | 67.7 ± 28.9 | 68.5 ± 31.2 | 67.0 ± 26.5 |
| Creatinine clearance‐no. (%) | |||
| <15 mL/min | 3 (0.03) | 2 (0.04) | 1 (0.02) |
| 15 to < 30 mL/min | 312 (2.8) | 131 (2.4) | 181 (3.1) |
| 30 to < 50 mL/min | 2382 (21.1) | 1099 (20.4) | 1283 (21.7) |
| 50 to < 80 mL/min | 4792 (42.4) | 2278 (42.2) | 2514 (42.5) |
| ≥80 mL/min | 2895 (25.6) | 1428 (26.5) | 1467 (24.8) |
| Unknown | 924 (8.2) | 458 (8.5) | 466 (7.9) |
| CHADS2 score | 2.2 ± 1.3 | 2.1 ± 1.3 | 2.3 ± 1.3 |
| CHADS2 score‐no. (%) | |||
| 0 | 985 (8.7) | 498 (9.2) | 487 (8.2) |
| 1 | 2802 (24.8) | 1439 (26.7) | 1363 (23.1) |
| 2 | 3400 (30.1) | 1701 (31.5) | 1699 (28.7) |
| 3 | 2206 (19.5) | 951 (17.6) | 1255 (21.2) |
| 4 | 1294 (11.4) | 532 (9.9) | 762 (12.9) |
| 5 | 514 (4.5) | 223 (4.1) | 291 (4.9) |
| 6 | 107 (0.9) | 52 (1.0) | 55 (0.9) |
| Baseline comorbidities‐no. (%) | |||
| Hypertension | 8405 (74.3) | 4094 (75.9) | 4311 (72.9) |
| Diabetes mellitus | 2523 (22.3) | 1182 (21.9) | 1341 (22.7) |
| Previous stroke or transient ischemic attack | 2675 (23.7) | 995 (18.4) | 1680 (28.4) |
| Congestive heart failure | 2826 (25.0) | 1351 (25.0) | 1475 (24.9) |
| Switch from other anticoagulants‐no. (%) | |||
| No | 5291 (46.8) | 2380 (44.1) | 2911 (49.2) |
| Yes | 6017 (53.2) | 3016 (55.9) | 3001 (50.8) |
| Warfarin | 3960 (35.0) | 1909 (35.4) | 2051 (34.7) |
| Dabigatran | 1688 (14.9) | 915 (17.0) | 773 (13.1) |
| Other | 369 (3.3) | 192 (3.6) | 177 (3.0) |
Plus‐minus values are means ±SD.
Figure 2.
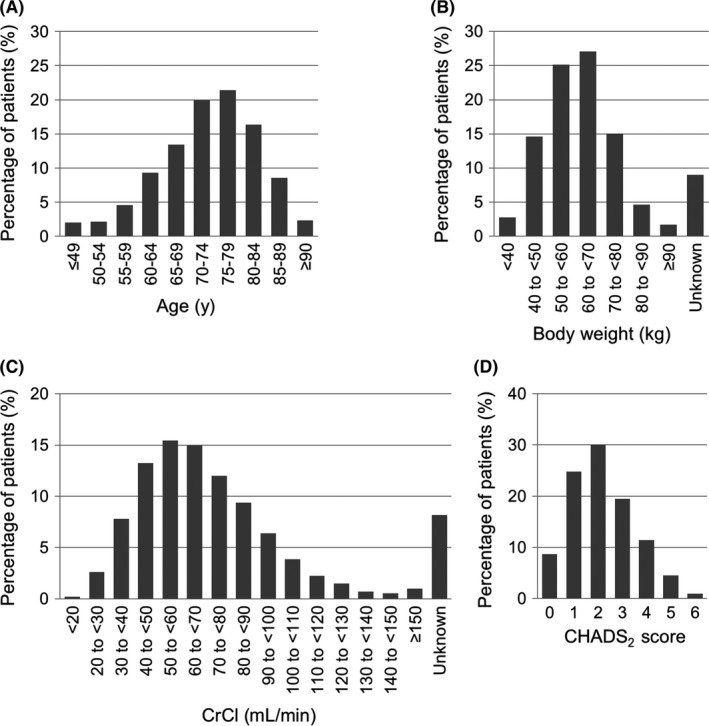
Patient distributions in different (A) age, (B) body weight, (C) CrCl, and (D) CHADS 2 score groups. CrCl, creatinine clearance
3.2. CHADS2 score in age or body weight groups
The CHADS2 score varied among age‐groups (Figure 3A, Figure S1A). More than 50% of <75‐year‐old patients had a CHADS2 score of 0‐1. More patients aged ≥75 than <75 years had a CHADS2 score of ≥2, which is partially caused by the fact that an age of ≥75 years is a risk factor for a higher CHADS2 score. Conversely, the CHADS2 score tended to gradually increase as body weight decreased (Figure 3B, Figure S1B). More than 70% of patients with a body weight of <50 kg had a CHADS2 score of ≥2.
Figure 3.
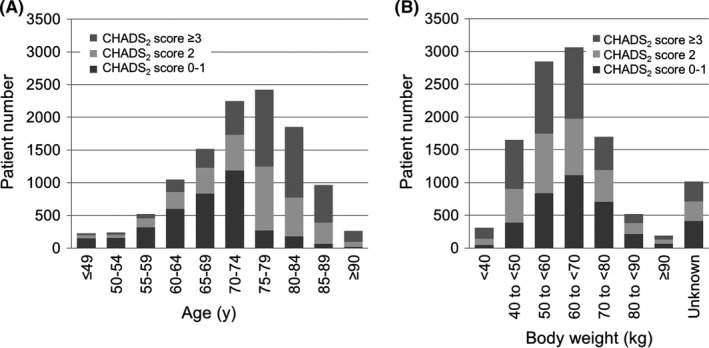
CHADS 2 score in different (A) age and (B) body weight groups
3.3. Age and body weight
Patients with a body weight of ≤50 kg or age of ≥75 years are considered to have a high risk of bleeding and must be carefully observed in the XAPASS as required by the Japanese health authority. Among the 11 308 patients enrolled in the XAPASS, 19.5% had a body weight of ≤50 kg and 48.7% had age of ≥75 years (Table 1). As shown in Figure 4, 1641 patients (14.5%) had a body weight of ≤50 kg and age of ≥75 years.
Figure 4.
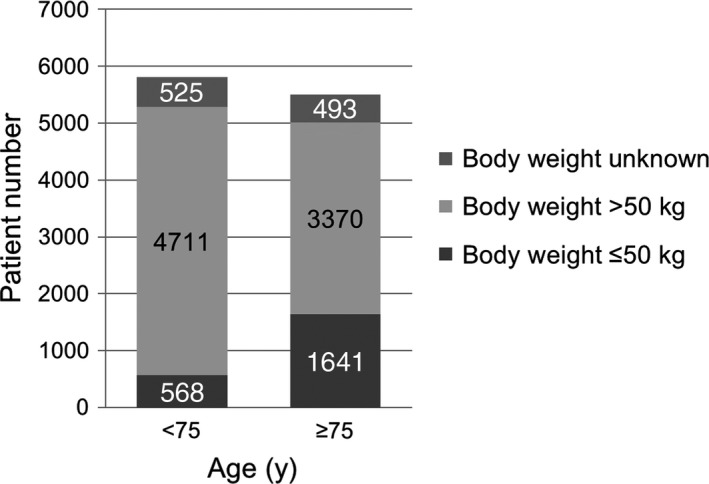
Distribution of body weight in different age‐groups. The patient number is described on each bar
3.4. CrCl in age, body weight, or CHADS2 score groups
The CrCl was examined in different age‐groups (Figure 5, Figure S2). As age increased, the percentage of patients with a low CrCl increased. Approximately half of patients aged 80‐84 years had a CrCl of <50 mL/min. The CrCl was also examined in different body weight groups (Figure S3A, B) and CHADS2 score groups (Figure S3C, D).
Figure 5.
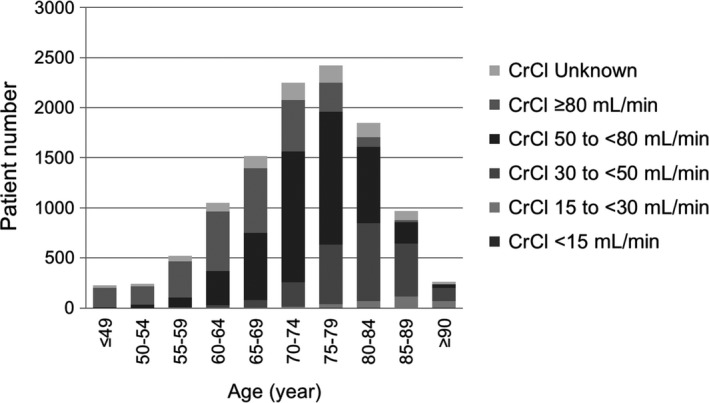
CrCl in different age‐groups. CrCl, creatinine clearance
4. DISCUSSION
The XAPASS is one of several postauthorization studies designed to investigate the safety and effectiveness of rivaroxaban in patients with NVAF in the real‐world clinical setting among different global regions. Outside Japan, the Xarelto for Prevention of Stroke in Patients with Atrial Fibrillation (XANTUS) program is in progress.10 The XANTUS program comprises four studies: the XANTUS (European Union, plus enrollment in Canada; NCT01606995), XANTUS‐EL (Eastern Europe, Eastern Mediterranean, Middle East, Latin America; NCT01800006), XANAP (Asia‐Pacific; NCT01750788), and XANTUS‐CN (People's Republic of China). The XANTUS (NCT01606995) revealed a low real‐world stroke incidence in patients receiving rivaroxaban, with an annual stroke rate of 0.7% (compared with 1.7 events per 100 patient‐years in the ROCKET AF on‐treatment population) and an incidence rate of major bleeding of 2.1 events per 100 patient‐years, which is lower than that in the ROCKET AF (3.6 events per 100 patient‐years).11 Other ongoing noninterventional registries also provide real‐world data on the effectiveness and safety of rivaroxaban, including the Global Anticoagulant Registry in the FIELD (GARFIELD)‐AF,12 Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT)‐AF,13 and the Dresden NOAC Registry.14
Additional information on the real‐world use of rivaroxaban in patients with NVAF will be provided in the XAPASS. For instance, the study will reveal real‐world situations of nonrecommended use such as over‐ or underdosing of rivaroxaban with respect to renal function. In Japan, the regular dosage of rivaroxaban is 15 mg od, which is lower than the global recommended dosage of 20 mg od. The Japanese medical package insert states that patients with a CrCl of ≥50 mL/min (preserved renal function) should be prescribed rivaroxaban 15 mg od and that patients with a CrCl of 15‐49 mL/min (moderate or severe renal impairment) should be prescribed a dosage of 10 mg od. In real‐world clinical practice, however, attending physicians usually determine the drug dosage for each patient based on the patient's characteristics and the physician's clinical experience, alongside the requirements in the medical package insert. In the XANTUS, 15% of 3812 patients with a documented CrCl of ≥50 mL/min received the lower rivaroxaban dosage of 15 mg od (the global reduced dosage for patients with NVAF with moderate or severe renal impairment); conversely, a dosage of 20 mg od (the global recommended dosage for patients with NVAF with preserved renal function) was received by 36% of the 640 patients who had moderate or severe renal impairment.11 Initial analysis of the XAPASS also showed that treatment was started at a lower rivaroxaban dosage of 10 mg od in 252 (50.8%) of 496 patients whose CrCl was ≥50 mL/min.15 The effects of these nonrecommended uses of rivaroxaban on safety and effectiveness outcomes will be reported.
Treatment persistence with rivaroxaban will also be revealed in the XAPASS. Treatment persistence is a major concern in stroke prevention because discontinuation of anticoagulation therapy affects the stroke risk in patients with AF.16 Persistence with rivaroxaban in the XANTUS was 80% at 1 year.11 This is higher than in recent US studies 17, 18 but in line with the Dresden NOAC Registry, in which discontinuations of approximately 15% were recorded in the first year.19
Evaluation of effectiveness and safety of Xa inhibitor for the Prevention of stroke And systemic embolism in a Nationwide cohort of Japanese patients Diagnosed as non‐valvular atrial fibrillation (EXPAND; UMIN000009376) study20 is ongoing and will also provide real‐world data on the effectiveness and safety of rivaroxaban. The EXPAND study is an investigator‐initiated clinical study based on a collaborative contract between Tohoku University Hospital and Bayer Yakuhin Ltd., which had no role in the study design, conduct of the study, data collection, data analysis, or preparation or submission of the manuscript. The main objective of the EXPAND study was to reveal the effectiveness and safety of rivaroxaban among Japanese patients with AF, including patients who were not included in the J‐ROCKET AF (eg, patients with a CHADS2 score of 0 or 1), in real‐world clinical practice. The XAPASS is a real‐world, prospective, observational study mandated by the Japanese authority as postmarketing surveillance and conducted by Bayer Yakuhin Ltd. under the supervision of a steering committee. The main objective of the XAPASS was to confirm the safety profile of rivaroxaban in real‐world use in Japan across a broad range of patients with NVAF through collection of AEs. Despite the differences in the study background/design and objective between the EXPAND study and XAPASS, the results of these studies will complement and strengthen each other as well as those of the phase III J‐ROCKET AF.
We herein report the baseline characteristics of the 11 308 patients enrolled in the XAPASS, which were clearly different from those of the patients enrolled in the J‐ROCKET AF. The low‐risk patients with a CHADS2 score of 0 or 1 were excluded from the J‐ROCKET AF8; in contrast, approximately one‐third of patients enrolled in the XAPASS had a CHADS2 score of 0 (8.7%) or 1 (24.8%). The distributions of the CHADS2 score were similar between the XAPASS and EXPAND study,20 suggesting that these results reflect the prescription pattern of rivaroxaban in Japan. It is unclear why a large number of patients with a CHADS2 score of 0 and 1 were prescribed rivaroxaban despite the fact that the Japanese guideline recommends rivaroxaban for patients with a CHADS2 score of ≥2. A recent subanalysis of the J‐RHYTHM Registry suggested that patients with lower CHADS2 scores benefit from anticoagulation,21 which might lead to the prescription of rivaroxaban for such patients. Compared with the XAPASS, the percentage of patients with a CHADS2 score of 0 or 1 was higher in other AF registries such as the J‐RHYTHM Registry (49.6%)22 and the SAKURA AF Registry (43.3%).23 This might be explained by the fact that the Japanese guideline recommends rivaroxaban for high‐risk patients with a CHADS2 score of ≥2.
There are some limitations to the XAPASS because of its open‐label, single‐arm, prospective, observational design. First, the open‐label nature of the study means that selection bias cannot be excluded because patients were enrolled with prior knowledge of rivaroxaban treatment, which was administered at their physician's discretion. Second, because the study design is single‐arm and therefore has no comparator drug such as warfarin, comparisons of different treatments are not possible. Finally, the observational design means that interference with patient management activities, such as further laboratory or other investigations (eg, of CrCl), was not permitted.
Strengths of the XAPASS include its large sample size (11 308 patients compared with 639 in the rivaroxaban arm of the J‐ROCKET AF)8 and prospective design, which allows for greater completeness of and potentially higher quality data than studies with retrospective designs.
The XAPASS is one of the largest AF registries in Japan. The 2‐year standard observation period ended in June 2016, and the maximum 5‐year follow‐up investigation will be completed in 2019. The incidence of safety and effectiveness outcomes in patients with NVAF treated with rivaroxaban in real‐world clinical practice in Japan will be clarified, and data on rivaroxaban use in a broad range of patients will be available in follow‐up studies. These include low‐ and high‐risk patients, such as those with a CHADS2 score of 0 or 1, weighing ≤50 kg, and age of ≥75 years. These data will supplement those from clinical trials, further clarifying optimal rivaroxaban use in Japanese patients, and dissemination of the XAPASS findings to clinical settings will be recommended.
5. CONCLUSIONS
The XAPASS provides practical information for the optimum use of rivaroxaban for stroke prevention in Japanese patients with AF in real‐world clinical settings and supplements the findings of the J‐ROCKET AF.
CONFLICT OF INTEREST
SO declares no conflict of interest. TI received lecture remuneration from Daiichi Sankyo, Ono Pharma, Mitsubishi‐Tanabe Pharma, Bayer Yakuhin, Bristol‐Myers Squibb, and Pfizer and scholarship funding from Daiichi Sankyo, Bristol‐Myers Squibb, Medtronic Japan, and St. Jude Medical. TK received lecture remuneration and scholarship funding from Bayer Yakuhin. JN received scholarship funding from Nihon Medi‐Physics. KM received lecture remuneration from Bayer Yakuhin and Otsuka Pharma. SM received scholarship funding from Takeda Pharma, CSL Behring, Meiji Seika Pharma, MSD, Astellas Pharma, Eisai, Otsuka Pharma, Carl Zeiss Meditec, Philips Electronics Japan, Sanofi, Siemens Healthcare, Daiichi Sankyo, Mitsubishi‐Tanabe Pharma, Chugai Pharma, Nihon Medi‐Physics, Pfizer, Bristol‐Myers Squibb, Brainlab, and Mizuho. YM received lecture remuneration and scholarship funding from Bayer Yakuhin. Y. Ohashi, MT, Y. Okayama, SY, and LI are employees of Bayer Yakuhin Ltd.
Supporting information
ACKNOWLEDGEMENTS
The authors acknowledge Ms. Sally Alexandroff (Bayer Pharma AG), who provided English editing support. The authors also thank Angela Morben, DVM, ELS, from Edanz Group (http://www.edanzediting.com/ac), for editing a draft of this manuscript.
APPENDIX A.
A.1.
The steering committee members are as follows:
Satoshi Ogawa (International University of Health & Welfare Mita Hospital, Tokyo, Japan); Takanori Ikeda (Department of Cardiovascular Medicine, Toho University Faculty of Medicine, Medical Center, Tokyo, Japan); Takanari Kitazono (Department of Medicine and Clinical Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan); Jyoji Nakagawara (Integrative Cerebral and Cardiovascular Imaging Center, National Cerebral and Cardiovascular Center, Suita, Osaka, Japan); Kazuo Minematsu (National Cerebral and Cardiovascular Center, Suita, Osaka, Japan); Susumu Miyamoto (Department of Neurosurgery, Kyoto University Graduate School of Medicine, Kyoto, Japan); and Yuji Murakawa (The 4th Department of Internal Medicine, Teikyo University School of Medicine, Mizonokuchi Hospital, Kawasaki, Japan).
Ogawa S, Minematsu K, Ikeda T, et al. Design and baseline characteristics of the Xarelto Post‐Authorization Safety & Effectiveness Study in Japanese Patients with Atrial Fibrillation (XAPASS). J Arrhythmia. 2018;34:167–175. https://doi.org/10.1002/joa3.12034
Funding information
The XAPASS is funded by Bayer Yakuhin Ltd. (Osaka, Japan).
REFERENCES
- 1. Inoue H, Fujiki A, Origasa H, et al. Prevalence of atrial fibrillation in the general population of japan: an analysis based on periodic health examination. Int J Cardiol. 2009;137:102–7. [DOI] [PubMed] [Google Scholar]
- 2. Akao M, Chun YH, Wada H, et al. Current status of clinical background of patients with atrial fibrillation in a community‐based survey: The fushimi af registry. J Cardiol. 2013;61:260–6. [DOI] [PubMed] [Google Scholar]
- 3. Akao M, Yamashita T, Okumura K, et al. Study design of J‐ELD AF: a multicenter prospective cohort study to investigate the efficacy and safety of apixaban in Japanese elderly patients. J Cardiol. 2015;68:554–8. [DOI] [PubMed] [Google Scholar]
- 4. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the framingham study. Stroke. 1991;22:983–8. [DOI] [PubMed] [Google Scholar]
- 5. Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the esc guidelines for the management of atrial fibrillation: an update of the 2010 esc guidelines for the management of atrial fibrillation. Developed with the special contribution of the european heart rhythm association. Eur Heart J. 2012;33:2719–47. [DOI] [PubMed] [Google Scholar]
- 6. Heidbuchel H, Verhamme P, Alings M, et al. Updated european heart rhythm association practical guide on the use of non‐vitamin k antagonist anticoagulants in patients with non‐valvular atrial fibrillation. Europace. 2015;17:1467–507. [DOI] [PubMed] [Google Scholar]
- 7. Perzborn E, Strassburger J, Wilmen A, et al. In vitro and in vivo studies of the novel antithrombotic agent bay 59‐7939–an oral, direct factor xa inhibitor. J Thromb Haemost. 2005;3:514–21. [DOI] [PubMed] [Google Scholar]
- 8. Hori M, Matsumoto M, Tanahashi N, et al. Rivaroxaban vs. Warfarin in japanese patients with atrial fibrillation ‐ the J‐ROCKET AF study. Circ J. 2012;76:2104–11. [DOI] [PubMed] [Google Scholar]
- 9. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 10. Camm AJ, Amarenco P, Haas S, et al. XANTUS: rationale and design of a noninterventional study of rivaroxaban for the prevention of stroke in patients with atrial fibrillation. Vasc Health Risk Manag. 2014;10:425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Camm AJ, Amarenco P, Haas S, et al. XANTUS: a real‐world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J. 2016;37:1145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kakkar AK, Mueller I, Bassand JP, et al. International longitudinal registry of patients with atrial fibrillation at risk of stroke: Global anticoagulant registry in the field (GARFIELD). Am Heart J. 2012;163:e1. [DOI] [PubMed] [Google Scholar]
- 13. Piccini JP, Fraulo ES, Ansell JE, et al. Outcomes registry for better informed treatment of atrial fibrillation: rationale and design of ORBIT‐AF. Am Heart J. 2011;162:e1. [DOI] [PubMed] [Google Scholar]
- 14. Beyer‐Westendorf J, Gelbricht V, Forster K, et al. Peri‐interventional management of novel oral anticoagulants in daily care: results from the prospective Dresden NOAC registry. Eur Heart J. 2014;35:1888–96. [DOI] [PubMed] [Google Scholar]
- 15. Ogawa S, Ikeda T, Kitazono T, et al. Present profiles of novel anticoagulant use in Japanese patients with atrial fibrillation: insights from the Rivaroxaban Postmarketing Surveillance Registry. J Stroke Cerebrovasc Dis. 2014;23:2520–6. [DOI] [PubMed] [Google Scholar]
- 16. Suzuki T, Shiga T, Omori H, et al. Adherence to medication and characteristics of Japanese patients with non‐valvular atrial fibrillation. J Cardiol. 2017;70:238–43. [DOI] [PubMed] [Google Scholar]
- 17. Laliberte F, Cloutier M, Nelson WW, et al. Real‐world comparative effectiveness and safety of rivaroxaban and warfarin in nonvalvular atrial fibrillation patients. Curr Med Res Opin. 2014;30:1317–25. [DOI] [PubMed] [Google Scholar]
- 18. Nelson WW, Song X, Coleman CI, et al. Medication persistence and discontinuation of rivaroxaban versus warfarin among patients with non‐valvular atrial fibrillation. Curr Med Res Opin. 2014;30:2461–9. [DOI] [PubMed] [Google Scholar]
- 19. Beyer‐Westendorf J, Forster K, Ebertz F, et al. Drug persistence with rivaroxaban therapy in atrial fibrillation patients‐results from the dresden non‐interventional oral anticoagulation registry. Europace. 2015;17:530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ikeda T, Atarashi H, Inoue H, et al. Study Design and Baseline Characteristics of the EXPAND Study: evaluation of Effectiveness and Safety of Xa Inhibitor, Rivaroxaban for the Prevention of Stroke and Systemic Embolism in a Nationwide Cohort of Japanese Patients Diagnosed as Non‐Valvular Atrial Fibrillation. Tohoku J Exp Med. 2016;240:259–68. [DOI] [PubMed] [Google Scholar]
- 21. Chishaki A, Kumagai N, Takahashi N, et al. Non‐valvular atrial fibrillation patients with low CHADS2 scores benefit from warfarin therapy according to propensity score matching subanalysis using the J‐RHYTHM Registry. Thromb Res. 2015;136:267–73. [DOI] [PubMed] [Google Scholar]
- 22. Atarashi H, Inoue H, Okumura K, et al. Present status of anticoagulation treatment in Japanese patients with atrial fibrillation: a report from the J‐RHYTHM Registry. Circ J. 2011;75:1328–33. [DOI] [PubMed] [Google Scholar]
- 23. Okumura Y, Yokoyama K, Matsumoto N, et al. Current use of direct oral anticoagulants for atrial fibrillation in Japan: findings from the SAKURA AF Registry. J Arrhythm. 2017;33:289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


