Figure EV3. Phosphorylation assays with Ubl and Ub mutants confirm the PINK1 binding site.
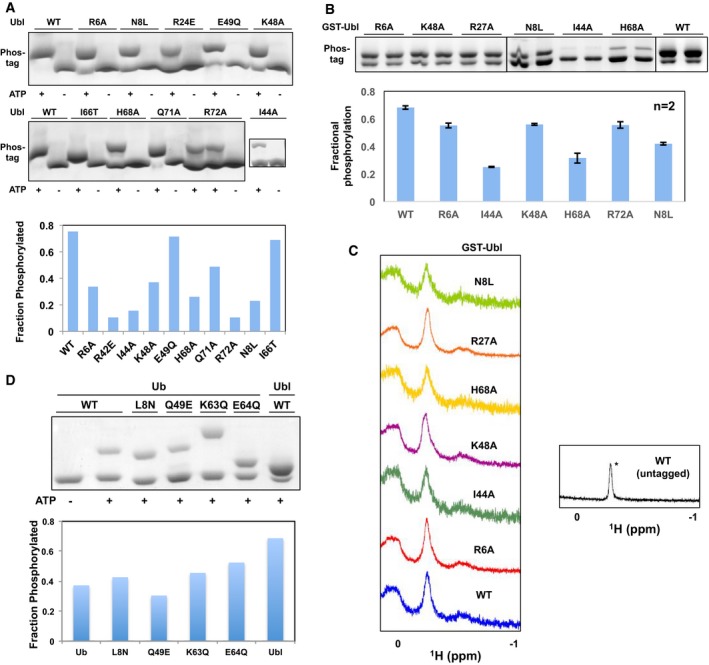
- (Left) Phos‐tag gels of phosphorylation assays shown in Fig 1D. (Right) Fractional levels of phosphorylation of WT and point mutants of Ubl (left). The phosphorylation assays were performed with 30 μM of GST‐Ubl and 2 μM GST‐TcPINK1143–570 and loaded on phos‐tag gels followed by densitometry (n = 1).
- (Top) Phos‐tag gels of phosphorylation assays and (bottom) fractional levels of phosphorylation of WT and point mutants of Ubl. The phosphorylation assays were performed with 12.5 μM of GST‐Ubl and 2 μM GST‐TcPINK1121–570 and loaded on phos‐tag gels followed by densitometry (n = 2). Error bars represent mean ± range of two independent phosphorylation experiments, loaded on the same gel.
- 1D proton NMR spectra of 30 μM GST‐Ubl WT or mutants, or untagged Ubl showing the characteristic Leu61 peak at −0.2 ppm (*) observed in all the mutants shown.
- (Top) Phos‐tag gels of phosphorylation assays and (bottom) fractional levels of phosphorylation of mutants of point of Ub made by mutating residues in Ub to corresponding residues in Ubl. The assays were conducted with 30 μM of GST‐Ubl and 0.5 μM GST‐TcPINK1121–570 for 30 min (n = 1).
