Fig. 7.
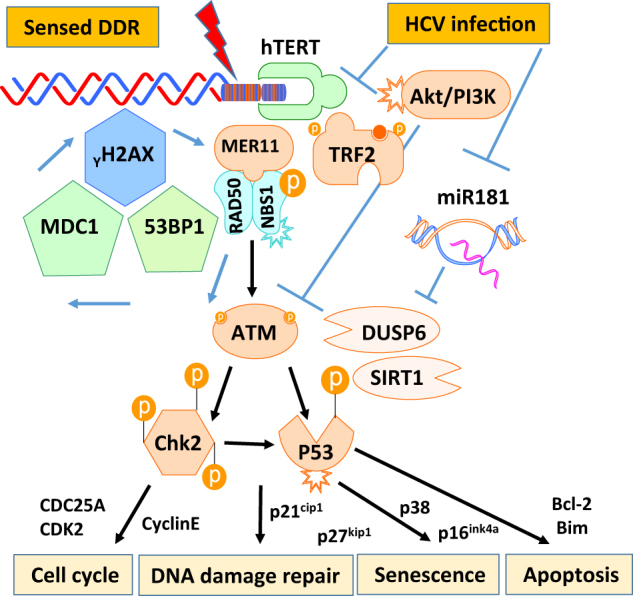
A novel model of HCV-induced ATM deficiency in T-cell cycle arrest, DNA damage repair, cell senescence, and apoptosis. HCV infection triggers a DNA damage response (DDR) in the early phase via activation of MRN-ATM-CHK2 and P53 signaling pathways in naive CD4 T cells, prompting cell cycle arrest and allowing for DNA damage repair; or, if the infection is overwhelming and causes unrepairable DNA damage, the cell will commit suicide and initiates programmed cell death (apoptosis). Persistent antigenic and inflammatory stimulation, however, drives ATM exhaustion and insufficiency, leading to impaired DNA damage repair and accumulation of DNA double strain breaks (DSBs), which result in constant cell apoptosis and naive T-cell loss. Excessive T-cell loss necessitates high homeostatic proliferation and imposes replicative stress on unprimed naive T cells, emerging as a novel molecular mechanism underlying T-cell senescence in the setting of chronic viral infection. Importantly, ectopic overexpression of ATM is necessary and sufficient to repair the DNA damage, survival deficit, and cellular dysfunction in HCV-derived T cells, providing a new strategy to improve immunotherapy and vaccine responses against human viral diseases
