Abstract
Background
To assess the association between final polytetrafluoroethylene (PTFE)-covered stent transjugular intrahepatic portosystemic shunt (TIPS) angiographic parameters and free shunt revision survey.
Methods
Series of two comparison groups were generated with persistence of varices or not, the 25th, 50th, and 75th percentile as cutoff for each angle and a 15-mm distance as cutoff for distance D. Kaplan Meier free shunt revision curves were then created and compared with Log Rank test.
Results
Mean follow-up was 455 days. Thirteen (19.4%) patients had shunt revision. Significant free shunt revision survey difference was found between post-procedural angiographic persistent varices group and the group without varices (P=0.0001). Shunt revision rate at 3, 12 and 24 months was respectively 13%, 29%, and 39% in the group with varices versus 0%, 2.7% and 2.7% in the group without. No difference was found between groups for angles A, B, C and distance D.
Conclusions
Persistence of gastric or esophageal varices on final trans-TIPS angiography increases TIPS revision rate after PTFE-covered stent shunt creation whereas geometric parameters have no influence.
Keywords: Transjugular intrahepatic portosystemic shunt (TIPS), gastric varices, oesophageal varices, covered stent, TIPS revision
Introduction
Transjugular intrahepatic portosystemic shunt (TIPS) is a safe and effective treatment (1,2) for the management of severe portal hypertension (3,4). Patients usually receive TIPS for three main indications: refractory ascites (5,6), variceal bleeding uncontrolled by medical and endoscopic treatment (7,8), and prevention from rebleeding for high-risk patients with first bleeding episode (early TIPS) (9-11). TIPS procedure is now well defined (12,13), with endovascular portal vein (PV) access via a sub-hepatic vein (SHV) approach, and then placement of a stent between the PV and the SHV. During the last decade, the use of polytetrafluoroethylene (PTFE) stent-grafts, alone or in combination with bare stents, has become the first-line choice for TIPS creation (14). Studies have demonstrated lower shunt revision rates and less recurrence of symptoms with covered stents in comparison with bare stents alone, with no obvious difference in terms of survival and hepatic encephalopathy (HE) (15-18). TIPS procedure can be performed by positioning two different stents, a bare stent and then a covered stent, or by placing a Viatorr® stent which is a combination of a bare stent and a covered stent, dedicated to TIPS.
The main complication of TIPS is the development of thrombosis or stenosis, and a better understanding of the risk factors which can lead to such complications is needed. Few studies have already been performed on the role of some geometric parameters as TIPS angulation and distance between TIPS and inferior vena cava, on TIPS patency, with conflicting results (19-23). This discrepancy between studies, and the little data available in the literature with the use of stent grafts led us to perform the present study.
The aim of this study was mainly to assess the association between final angiographic parameters and free shunt revision survey in patients treated with PTFE-covered stent TIPS.
Methods
Study population
Retrospective review of the medical records of patients who underwent TIPS procedure from January 2012 to September 2015 was conducted. Patients were identified using the database maintained prospectively by the Interventional Radiology Department. There were 67 patients overall, 58 males (86.6%) and 9 females (13.4%) with a mean age of 56.3±12.0 years (range, 26–77 years). Patients lost to follow-up (n=2) or with no available images for analysis (n=3) were excluded from the analysis. All patients suffered from cirrhosis, 52 (77.6%) from alcoholic cirrhosis, 8 (11.9%) from alcoholic liver disease associated with another cause of liver disease [viral, non-alcoholic steatohepatitis (NASH), or other cause of cirrhosis]. Patient’s characteristics are presented in Table 1. This retrospective study was performed in compliance with the requirements of the institutional review board and approved by the institution ethical committee. Informed consent was waived.
Table 1. Descriptive analysis of the study population of 67 patients.
| Characteristics | Variables |
|---|---|
| Patient characteristics | |
| Age (years), mean ± SD [range] | 56.3±12.0 [26–77] |
| Gender (male/female), n (%) | 58 (86.6)/9 (13.4) |
| Cause of cirrhosis, n (%) | |
| Alcohol | 52 (77.6) |
| Alcohol + other cause | 8 (11.9) |
| Viral hepatitis | 2 (3.0) |
| Other cause | 5 (7.5) |
| Child-Pugh class, n (%) | |
| A | 7 (10.5) |
| B | 51 (76.1) |
| C | 9 (13.4) |
| Indication for TIPS, n (%) | |
| Salvage TIPS | 6 (9.0) |
| Early TIPS | 24 (35.8) |
| Refractory ascites | 37 (55.2) |
| Procedure characteristics | |
| Geometric parameters, mean ± SD [range] | |
| Distance D (mm) | 10.0±9.6 [0–33.5] |
| Angle A (°) | 152.3±11.5 [114–174] |
| Angle B (°) | 132.3±30.0 [57.5–173] |
| Angle C (°) | 151.7±21.7 [76.5–179] |
| Angle M (°) | 145.4±11.6 [112–164.5] |
| Gastric or esophageal varices on final angiography, n (%) | 31 (46.3) |
| Type of stent, n (%) | |
| Nitinol uncovered stent + PTFE-covered stent | 57 (85.1) |
| Viatorr stent | 10 (14.9) |
| Stent diameter, n (%) | |
| 8 mm | 1 (1.5) |
| 10 mm | 63 (94.0) |
| 12 mm | 3 (4.5) |
| Sub-hepatic vein access, n (%) | |
| Right sub-hepatic vein | 58 (86.6) |
| Median or left sub-hepatic vein | 9 (13.4) |
| Number of TIPS per operator, n (%) | |
| Operator 1 | 7 (10.4) |
| Operator 2 | 39 (58.2) |
| Operator 3 | 5 (7.5) |
| Operator 4 | 16 (23.9) |
| Follow-up characteristics | |
| Follow-up duration (days), mean ± SD [range] | 455.1±307.9 [35–1,365] |
| Free-revision period (days), mean ± SD [range] | 414.9±301.3 [24–1,365] |
| Shunt revision rate, n (%) | 13 (19.4) |
| Mortality rate, n (%) | 15 (22.4) |
TIPS procedure
All procedures were performed under general anesthesia by four interventional radiologists with 20 (Jean-Pierre Cercueil), 12 (Romaric Loffroy), 5 (Marco Midulla) and 2 (Sophie Gehin) years of experience with TIPS procedures, respectively, as previously described in the literature according to the following standard schema: step 1, right internal jugular venous access; step 2, hepatic venography; step 3, wedged hepatic venography; step 4, accessing the PV, portography and measure of portosystemic gradient; step 5, dilatation of the parenchymal tract; step 6, deployment of a stent across the parenchymal tract; step 7, portography and measure of portosystemic gradient (24-26). More in detail, the “Ring TIPS” set (Cook Medical, Bloomington, IN, USA) with Colapinto needle was used in all patients. Only X-ray guidance was used for PV access. Different combinations of stent were used. Before December 2014, a combination of bare stent (WallstentTM, Boston Scientific, Natick, USA; or Protege GPSTM stent, Covidien, Dublin, Ireland) and PTFE-covered stent (Advanta V12TM, Maquet, Wayne, USA; or FluencyTM, Bard, New York, USA) was used for TIPS. After December 2014, the dedicated Viatorr® PTFE-covered stent (W.L. Gore & Associates, Newark, DE, USA) was used for TIPS, avoiding the use of two stents. The distal portal portion of this stent is uncovered whereas the medium parenchyma and proximal hepatic portions are covered. Catheterization of the PV with the 10-French TIPS sheath was required to place the Viatorr® PTFE-covered stent-graft between the PV and the hepatic vein. Sometimes, TIPS was created by deploying an uncovered metal stent first, through which the TIPS sheath is usually advanced into the PV. In these cases, the PTFE-covered stent is deployed within the bare-metal stent. When using dedicated Viatorr® PTFE-covered stent-graft, care should be taken to leave the uncovered caudal portion of the stent in the PV, whereas the covered portion of the stent should be in the parenchymal tract and in the hepatic vein. The cranial end of the stent should extend to the junction of the hepatic vein and the inferior vena cava. Overlapping stents of the same diameter were often used to achieve the desired shunt length and to reduce severe angulation within the shunt. The diameter of the covered stent used was 8 mm in 1 patient, 10 mm in 63 patients, or 12 mm in 3 patients. No primary adjunctive variceal embolotherapy was performed. Antibiotics were administered per-procedurally but no adjunctive anticoagulant or antiplatelet therapy was given.
Follow-up
Follow-up included clinical examination and TIPS Doppler ultrasound (US) after 2 days, and at 1, 3, and 6 months, and every 6 months thereafter. Doppler US was also performed in case of recurrence of symptoms. Doppler US velocimetry was considered normal between 90 and 180 cm/second (27,28). For patients with velocimetry values below 60 or above 180 cm/second, or patients with Doppler US signs of focal stenosis, or patients with recurrence of symptoms and undetermined Doppler US velocimetry, exploration was completed by TIPS angiography. A stenosis on TIPS angiography was defined as a 50% stenosis in two orthogonal views and/or portosystemic gradient greater than 12 mmHg. In such case, shunt’s revision was performed by angioplasty +/− additional stenting at the operator discretion. Stenting was the rule in case of complete thrombosis. The mean follow-up duration was 455.1±307.9 days (range, 35–1,365 days). The mean free shunt revision duration was 414.9±301.3 days (range, 24–1,365 days). Overall, 13 patients (19.4%) had a shunt revision and 15 patients (22.4%) died during the mean follow-up.
Data review
Angiographic images obtained during TIPS placement were reviewed by two radiologists, blinded to the outcomes. Digital subtraction angiographic images in anterior view were used for the measurement of the following geometric parameters: angle A (hepatic vein to parenchymal tract angle), angle B (intra-TIPS angle) and angle C (PV to stent angle) as shown in Figure 1. The angle M (mean of angle A, B and C) and the distance D (stent to hepato-caval junction distance) were also generated. All patients included in the study had a TIPS angiography that depicted the confluence of the inferior vena cava and the hepatic vein, and a trans-TIPS portal venography that depicted the confluence between splenomesenteric vein and the PV. The presence of gastric or oesophageal varices on final angiography was also determined by reviewing angiographic images. Filing of gastric or esophageal varices was defined by still viewing varicose vessel on the final trans-TIPS portal venography, with a diameter more than 5 mm, and peri-stomachal location according to the Sarin’s classification (29).
Figure 1.
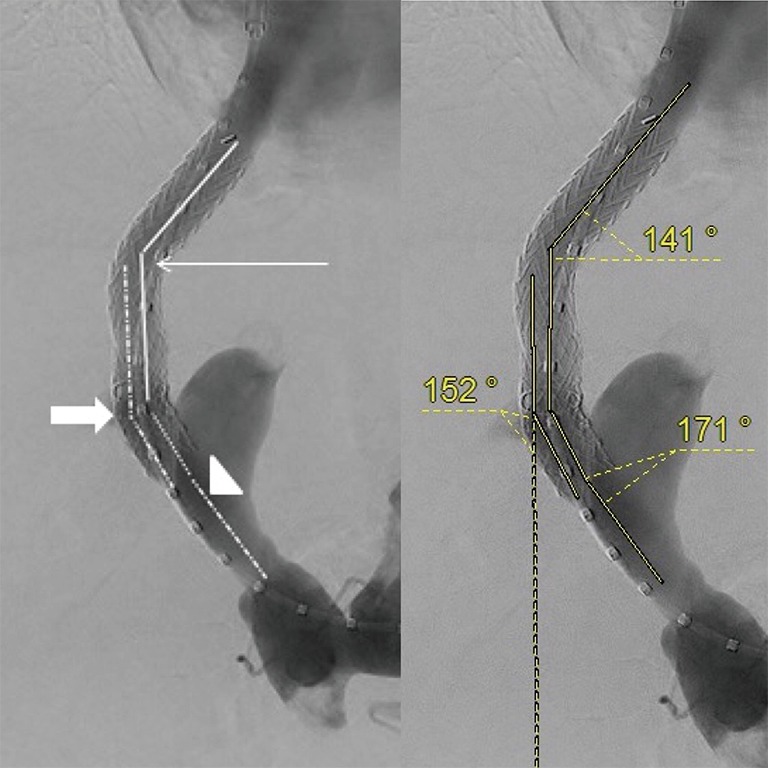
Example of angle A (thin arrow), angle B (head of arrow) and angle C (arrow), on the left, and equivalent value in degrees on the right.
Statistical analysis
Firstly, Kappa correlation coefficient study was performed to check the level of agreement between the two images reviewers. Then, Kaplan Meier curves were generated to compare the free TIPS revision survey and overall mortality according to baseline demographics and angiographic parameters. Each angle A, B, C and M were tested with the 25th, 50th, and 75th percentile as cutoff to generate two groups of comparison. The distance D was tested with a distance of 15 millimeters as cutoff. Kaplan Meier analysis curves were also created to compare Viatorr® stent alone versus combination of covered/bare stent, presence of varices on final angiography versus absence of varices, men versus women, right SHV access versus other access, Child-Pugh class C versus other class, alcoholic cirrhosis versus other cause of cirrhosis, salvage indication versus other indication. For each curve, we used Log Rank test with χ2 to determine whether free TIPS revision and survival curves were statistically significantly different. A value of P<0.05 was considered statistically significant. Multivariate analysis with Cox proportional hazard regression was not possible. Statistical analyses were performed using STATA version 14 (STATA Corp., Texas, USA).
Results
Rate of shunt revision
TIPS revision rate was 9% at 1 year (6 of 67 patients) and 13.4% at 2 years (9 of 67 patients).
Reviewers’ correlation
An excellent correlation was found between the two reviewers with Kappa correlation coefficient >90 for each angle (A, B, C and M) and for the distance D.
Parameters evaluation
Technical parameters
No significant difference of revision rate was found between the group with the Viatorr® stent and the group with a combination of graft and bare stents (χ2=3.46; P=0.063). No difference was found between the group of TIPS (n=39) performed by the most experienced operator and the group of TIPS (n=28) performed by other operators (χ2=2.11; P=0.63).
Clinical parameters
No statistically significant difference was found between the two groups of TIPS (revised versus non-revised) for the following demographic parameters: sex, etiology of cirrhosis, Child Pugh class, TIPS indication.
Geometric parameters
A statistically significant free shunt revision survey difference was found between the group of patients who had gastric or esophageal varices on angiographic control and the group of patients without varices (χ2=16.25; P=0.0001). Shunt revision rate at 3, 12 and 24 months was respectively 13%, 29%, and 39% in the group with varices, versus 0, 2.7%, and 2.7% in the group without varices. Kaplan Meier free TIPS revision curves are presented in Figure 2. The presence of varices on final angiography increased shunt revision rate of respectively 13%, 26.3% and 36.3% at 3, 12 and 24 months. No difference of revision rate was found between the group with right SHV access and the group with other SHV access (χ2=0.54; P=0.46). For each angle tested (angles A, B, C and M), no statistically significant difference of revision rate was found between the two groups, with the 25th, 50th, and 75th percentile as cutoff. No statistically difference was found between the group of patients with a stent to inferior vena cava distance ≤15 mm and the group of patients with a distance >15 mm (χ2=0.08; P=0.78).
Figure 2.
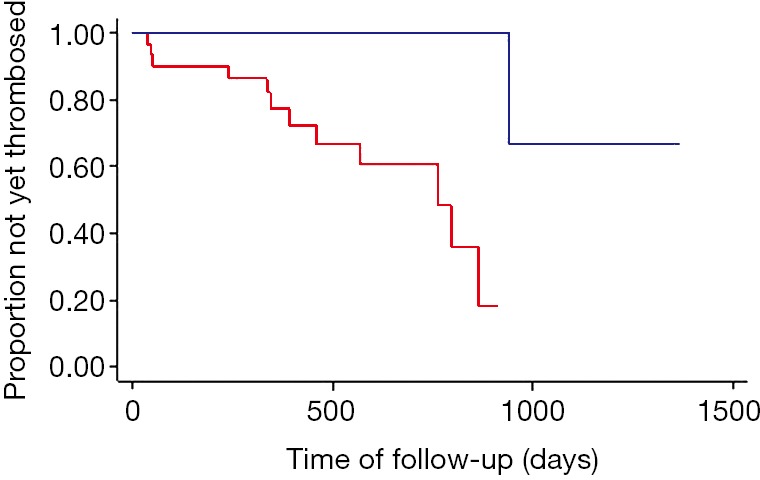
Comparison of free shunt revision survey between patients with (red curve) or without (blue curve) filling gastric or oesphageal varices on trans-TIPS final angiography. TIPS, transjugular intrahepatic portosystemic shunt.
Mortality
All cause-mortality rate was 16.4% at 1 year (11 of 67 patients) and 20.9% at 2 years (14 of 67 patients). There was no difference in survey between patients with the presence of varices at final angiography and patients without varices. No statistically significant difference was found for each Log rank test performed with technical, clinical, and geometric parameters.
Discussion
It has been shown that the use of the Viatorr® stents for TIPS results in a markedly prolonged shunt patency up to 76% at 2 years versus 8% to 48% at 2 years for TIPS created with bare metal stents (30,31). Early and late shunt dysfunction must be differentiated. Most of early occlusion events with bare stents are due to the development of biliary venous fistula, caused by the puncture with Cola Pinto needle or occurring during stent expansion. This kind of occlusion events are less common with graft stents because biliary injury is covered by the graft stent, and prevent from the biliary venous fistula development (32,33). Late TIPS stenoses, which may lead to occlusion, are mostly caused by intimal hyperplasia (34,35). The use of graft stents seems to be associated with lower intimal hyperplasia (36,37). The combination of those two factors probably explains why position of the vein end graft stent and then the distance between stent and inferior vena cava had no influence on the shunt patency in our study as in the study by Andring et al. (23). It suggests that positioning of the end vein graft stent at the junction of the ICV with SHV should not be a priority during TIPS procedure.
Our study found no relation between TIPS angulation and shunt revision rate or overall survey. This result is in accordance with other series, either with bare metal stents (19-21) or with graft stents as well (23). It is well-known that covered stents (Viatorr® stent or bare-metal stent + covered stent) give better outcomes and long-term shunt patency than uncovered bare metal stents alone, with no difference between dedicated Viatorr® stent and other covered stents (31).
In our study, persistence of esophageal or gastric varices on trans-TIPS angiographic control was associated with higher rate of shunt revision, without effect on mortality. At our knowledge, no other studies showed such data. This could be explained by the fact that oesophageal or gastric post-TIPS varices create a competitive flow, leading to more risk of stent thrombosis or stenosis because of lower flow into the TIPS (38,39). Indeed, persistent flow into varices can be responsible for hemodynamic steal from the PV leading to slower flow into the TIPS shunt and potentially higher risk of in-stent thrombosis. Few authors studied the role of esophageal or gastric varices embolization at the end of the TIPS procedure, but his role is not clearly defined nowadays. In a review of 166 patients who underwent TIPS for variceal bleeding (37), Lakhoo et al. found that most common causes of rebleeding were lack of or insufficient variceal embolization (64%). In a randomized control trial (38), Chen et al. found that the 6-month shunt patency rate was higher (96.2% vs. 82%, P=0.019) and the 6-month rebleeding rate was lower (5.7% vs. 20%, P=0.029) in the group “TIPS + variceal embolization” than in the group “TIPS alone”, whereas the 3-year cumulative rates of shunt patency, recurrent variceal bleeding, and death were not different between groups (P>0.05). A meta-analysis reported that patients with TIPS + variceal embolization had significantly lower rebleeding (OR=2.02, P=0.002) but similar incidences of shunt dysfunction, HE, and death than patients with TIPS alone (40). Furthermore, Xiao et al. (41) found no difference in incidence of rebleeding, shunt revision, encephalopathy, and overall survival in patients with portosystemic gradient ≤12 mmHg after stent implantation and angioplasty. However, portosystemic gradient after TIPS placement was an independent predictor of rebleeding (P=0.036).
Finally, there is no clear recommendation with high level of evidence on the role of embolization for varices management, although literature is rather in favor of a decrease of rebleeding rate after embolization, as found by Tesdal et al. (40). The effect on shunt patency or death remains to be proven. Whatever the type of procedure, every effort should be performed to ensure a portosystemic gradient ≤12 mmHg, with variceal embolization or not. Adjunctive embolization, when the post-TIPS portosystemic gradient is still ≥12 mmHg, appears to be attractive but only based on personal experience of such authors. This discrepancy supports the need for a randomized clinical trial, with clearly defined variceal embolization criteria, including portosystemic pressure gradient and the filling of varices on final trans-TIPS angiography.
In conclusion, in the present study, filling of esophageal or gastric varices >5 mm on the trans-TIPS final angiography was statistically associated with a higher shunt revision rate, without any impact on the global survey. No influence of demographic, geometric or technical parameters was found on the shunt revision rate and the global survey. This study suggests that variceal embolization during TIPS procedure could increase free shunt revision survey when final trans-TIPS angiography shows filling of esophageal or gastric varices ≥5 mm. Further studies are needed to confirm these data and define accurate indications of variceal embolization.
Acknowledgements
None.
Ethical Statement: This retrospective study was performed in compliance with the requirements of the institutional review board and approved by the institution ethical committee. Informed consent was waived.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Boyer TD, Haskal ZJ, American Association for the Study of Liver Diseases The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology 2010;51:306. 10.1002/hep.23383 [DOI] [PubMed] [Google Scholar]
- 2.Bari K, Garcia-Tsao G. Treatment of portal hypertension. World J Gastroenterol 2012;18:1166-75. 10.3748/wjg.v18.i11.1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fidelman N, Kwan SW, LaBerge JM, Gordon RL, Ring EJ, Kerlan RK. The transjugular intrahepatic portosystemic shunt: an update. AJR Am J Roentgenol 2012;199:746-55. 10.2214/AJR.12.9101 [DOI] [PubMed] [Google Scholar]
- 4.Boyer TD, Haskal ZJ. American Association for the Study of Liver Diseases Practice Guidelines: the role of transjugular intrahepatic portosystemic shunt creation in the management of portal hypertension. J Vasc Interv Radiol 2005;16:615-29. 10.1097/01.RVI.0000157297.91510.21 [DOI] [PubMed] [Google Scholar]
- 5.de Franchis R, Baveno VI. Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010;53:762-8. 10.1016/j.jhep.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 6.de Franchis R, Baveno VI. Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:743-52. 10.1016/j.jhep.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 7.Azoulay D, Castaing D, Majno P, Saliba F, Ichaï P, Smail A, Delvart V, Danaoui M, Samuel D, Bismuth H. Salvage transjugular intrahepatic portosystemic shunt for uncontrolled variceal bleeding in patients with decompensated cirrhosis. J Hepatol 2001;35:590-7. 10.1016/S0168-8278(01)00185-4 [DOI] [PubMed] [Google Scholar]
- 8.Loffroy R, Estivalet L, Cherblanc V, Favelier S, Pottecher P, Hamza S, Minello A, Hillon P, Thouant P, Lefevre PH, Krausé D, Cercueil JP. Transjugular intrahepatic portosystemic shunt for the management of acute variceal hemorrhage. World J Gastroenterol 2013;19:6131-43. 10.3748/wjg.v19.i37.6131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Pagán JC, Di Pascoli M, Caca K, Laleman W, Bureau C, Appenrodt B, Luca A, Zipprich A, Abraldes JG, Nevens F, Vinel JP, Sauerbruch T, Bosch J. Use of early-TIPS for high-risk variceal bleeding: results of a post-RCT surveillance study. J Hepatol 2013;58:45-50. 10.1016/j.jhep.2012.08.020 [DOI] [PubMed] [Google Scholar]
- 10.Rudler M, Cluzel P, Corvec TL, Benosman H, Rousseau G, Poynard T, Thabut D. Early-TIPS placement prevents rebleeding in high-risk patients with variceal bleeding, without improving survival. Aliment Pharmacol Ther 2014;40:1074-80. 10.1111/apt.12934 [DOI] [PubMed] [Google Scholar]
- 11.Halabi SA, Sawas T, Sadat B, Jandali A, Halabi HA, Halabi FA, Kapoor B, Carey WD. Early TIPS versus endoscopic therapy for secondary prophylaxis after management of acute esophageal variceal bleeding in cirrhotic patients: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol 2016;31:1519-26. 10.1111/jgh.13303 [DOI] [PubMed] [Google Scholar]
- 12.Clark TW. Stepwise placement of a transjugular intrahepatic portosystemic shunt endograft. Tech Vasc Interv Radiol 2008;11:208-11. 10.1053/j.tvir.2009.04.002 [DOI] [PubMed] [Google Scholar]
- 13.Loffroy R, Favelier S, Pottecher P, Estivalet L, Genson PY, Gehin S, Krausé D, Cercueil JP. Transjugular intrahepatic portosystemic shunt for acute variceal gastrointestinal bleeding: Indications, techniques and outcomes. Diagn Interv Imaging 2015;96:745-55. 10.1016/j.diii.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 14.Maleux G, Nevens F, Wilmer A, Heye S, Verslype C, Thijs M, Wilms G. Early- and long-term clinical and radiological follow-up results of expanded-polytetrafluoroethylene-covered stent-grafts for transjugular intrahepatic portosystemic shunt procedures. Eur Radiol 2004;14:1842-50. 10.1007/s00330-004-2359-4 [DOI] [PubMed] [Google Scholar]
- 15.Cejna M, Peck-Radosavljevic M, Thurnher SA, Hittmair K, Schoder M, Lammer J. Creation of transjugular intrahepatic portosystemic shunts with stent-grafts: initial experience with a polytetrafluoroethylene-covered nitinol endoprosthesis. Radiology 2001;221:437-46. 10.1148/radiol.2212010195 [DOI] [PubMed] [Google Scholar]
- 16.Saxon RR. A new era for transjugular intrahepatic portosystemic shunts? J Vasc Interv Radiol 2004;15:217-9. 10.1097/01.RVI.0000116862.34422.A5 [DOI] [PubMed] [Google Scholar]
- 17.Bureau C, Garcia-Pagan JC, Otal P, Pomier-Layrargues G, Chabbert V, Cortez C, Perreault P, Péron JM, Abraldes JG, Bouchard L, Bilbao JI, Bosch J, Rousseau H, Vinel JP. Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: results of a randomized study. Gastroenterology 2004;126:469-75. 10.1053/j.gastro.2003.11.016 [DOI] [PubMed] [Google Scholar]
- 18.Rossi P, Salvatori FM, Fanelli F, Bezzi M, Rossi M, Marcelli G, Pepino D, Riggio O, Passariello R. Polytetrafluoroethylene-covered nitinol stent-graft for transjugular intrahepatic portosystemic shunt creation: 3-year experience. Radiology 2004;231:820-30. 10.1148/radiol.2313030349 [DOI] [PubMed] [Google Scholar]
- 19.Schaefer PJ, Jahnke T, Schaefer FK, Hedderich J, Hinrichsen H, Heller M, Müller-Hülsbeck S. Transjugular intrahepatic portosystemic shunt: evaluation of the impact of the stent’s configuration on the patency rate. Rofo 2007;179:965-70. 10.1055/s-2007-963394 [DOI] [PubMed] [Google Scholar]
- 20.Perry LJ, Amatulle P, Brophy DP, Lang EV. Does the geometry of TIPS predict the likelihood of subsequent shunt dysfunction? J Vasc Interv Radiol 2000;11:S297. [Google Scholar]
- 21.Weeks SM, Rehder K, Sandhu J, Shrestha R, Jaques PF, Mauro MA. TIPS: does shunt geometry affect patency? J Vasc Interv Radiol 2001;12:S138. [Google Scholar]
- 22.Clark TW, Agarwal R, Haskal ZJ, Stavropoulos SW. The effect of initial shunt outflow position on patency of transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol 2004;15:147-52. 10.1097/01.RVI.0000109401.52762.56 [DOI] [PubMed] [Google Scholar]
- 23.Andring B, Kalva SP, Sutphin P, Srinivasa R, Anene A, Burrell M, Xi Y, Pillai AK. Effect of technical parameters on transjugular intrahepatic portosystemic shunts utilizing stent grafts. World J Gastroenterol 2015;21:8110-7. 10.3748/wjg.v21.i26.8110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loffroy R. Transjugular intrahepatic portosystemic shunt (TIPS): A major step forward for patients but a growing job for interventional radiologists! Diagn Interv Imaging 2016;97:1069-70. 10.1016/j.diii.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 25.Rouabah K, Varoquaux A, Caporossi JM, Louis G, Jacquier A, Bartoli JM, Moulin G, Vidal V. Image fusion-guided portal vein puncture during transjugular intrahepatic portosystemic shuntplacement. Diagn Interv Imaging 2016;97:1095-102. 10.1016/j.diii.2016.06.015 [DOI] [PubMed] [Google Scholar]
- 26.Borghol S, Perarnau JM, Pucheux J, D'Alteroche L, Ayoub J, Trillaud H. Short- and long-term evolution of the endoluminal diameter of underdilated stents in transjugular intrahepatic portosystemic shunt. Diagn Interv Imaging 2016;97:1103-7. 10.1016/j.diii.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 27.Ferguson JM, Jalan R, Redhead DN, Hayes PC, Allan PL. The role of duplex and colour Doppler ultrasound in the follow-up evaluation of transjugular intrahepatic portosystemic stent shunt (TIPSS). Br J Radiol 1995;68: 587-9. 10.1259/0007-1285-68-810-587 [DOI] [PubMed] [Google Scholar]
- 28.Kanterman RY, Darcy MD, Middleton WD, Sterling KM, Teefey SA, Pilgram TK. Doppler sonography findings associated with transjugular intrahepatic portosystemic shunt malfunction. AJR Am J Roentgenol 1997;168:467-72. 10.2214/ajr.168.2.9016228 [DOI] [PubMed] [Google Scholar]
- 29.Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: A long-term follow-up study in 568 portal hypertension patients. Hepatology 1992;16:1343-9. 10.1002/hep.1840160607 [DOI] [PubMed] [Google Scholar]
- 30.Jirkovsky V, Fejfar T, Safka V, Hulek P, Krajina A, Chovanec V, Raupach J, Lojik M, Vanasek T, Renc O, Ali SM. Influence of the secondary deployment of expanded polytetrafluoroethylene-covered stent grafts on maintenance of transjugular intrahepatic portosystemic shunt patency. J Vasc Interv Radiol 2011;22:55-60. 10.1016/j.jvir.2010.09.016 [DOI] [PubMed] [Google Scholar]
- 31.Bureau C, Garcia Pagan JC, Layrargues GP, Metivier S, Bellot P, Perreault P, Otal P, Abraldes JG, Peron JM, Rousseau H, Bosch J, Vinel JP. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multicentre study. Liver Int 2007;27:742-7. 10.1111/j.1478-3231.2007.01522.x [DOI] [PubMed] [Google Scholar]
- 32.Jawaid Q, Saeed ZA, Di Bisceglie AM, Brunt EM, Ramrakhiani S, Varma CR, Solomon H. Biliary-venous fistula complicating transjugular intrahepatic portosystemic shunt presenting with recurrent bacteremia, jaundice, anemia and fever. Am J Transplant 2003;3:1604-7. 10.1046/j.1600-6135.2003.00267.x [DOI] [PubMed] [Google Scholar]
- 33.Willner IR, El-Sakr R, Werkman RF, Taylor WZ, Riely CA. A fistula from the portal vein to the bile duct: an unusual complication of transjugular intrahepatic portosystemic shunt. Am J Gastroenterol 1998;93:1952-5. 10.1111/j.1572-0241.1998.00553.x [DOI] [PubMed] [Google Scholar]
- 34.Ducoin H, El-Khoury J, Rousseau H, Barange K, Peron JM, Pierragi MT, Rumeau JL, Pascal JP, Vinel JP, Joffre F. Histopathologic analysis of transjugular intrahepatic portosystemic shunts. Hepatology 1997;25:1064-9. 10.1002/hep.510250503 [DOI] [PubMed] [Google Scholar]
- 35.Teng GJ, Bettmann MA, Hoopes PJ, Ermeling BL, Yang L, Wagner RJ. Transjugular intrahepatic portosystemic shunt in a porcine model: histologic characteristics at the early stage. Acad Radiol 1998;5:547-55. 10.1016/S1076-6332(98)80206-7 [DOI] [PubMed] [Google Scholar]
- 36.Huang Q, Wu X, Fan X, Cao J, Han J, Xu L, Li N. Comparison study of Doppler ultrasound surveillance of expanded polytetrafluoroethylene-covered stent versus bare stent in transjugular intrahepatic portosystemic shunt. J Clin Ultrasound 2010;38:353-60. [DOI] [PubMed] [Google Scholar]
- 37.Lakhoo J, Bui JT, Zivin SP, Lokken RP, Minocha J, Ray CE, Jr, Gaba RC. Root cause analysis of rebleeding events following transjugular intrahepatic portosystemic shunt creation for variceal hemorrhage. J Vasc Interv Radiol 2015;26:1444-53. 10.1016/j.jvir.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 38.Chen S, Li X, Wei B, Tong H, Zhang MG, Huang ZY, Cao JW, Tang CW. Recurrent variceal bleeding and shunt patency: prospective randomized controlled trial of transjugular intrahepatic portosystemic shunt alone or combined with coronary vein embolization. Radiology 2013;268:900-6. 10.1148/radiol.13120800 [DOI] [PubMed] [Google Scholar]
- 39.Qi X, Liu L, Bai M, Chen H, Wang J, Yang Z, Han G, Fan D. Transjugular intrahepatic portosystemic shunt in combination with or without variceal embolization for the prevention of variceal rebleeding: a meta-analysis. J Gastroenterol Hepatol 2014;29:688-96. 10.1111/jgh.12391 [DOI] [PubMed] [Google Scholar]
- 40.Tesdal IK, Filser T, Weiss C, Holm E, Dueber C, Jaschke W. Transjugular intrahepatic portosystemic shunts: adjunctive embolotherapy of gastroesophageal collateral vessels in the prevention of variceal rebleeding. Radiology 2005;236:360-7. 10.1148/radiol.2361040530 [DOI] [PubMed] [Google Scholar]
- 41.Xiao T, Chen L, Chen W, Xu B, Long Q, Li R, Li L, Peng Z, Fang D, Wang R. Comparison of transjugular intrahepatic portosystemic shunt (TIPS) alone versus TIPS combined with embolotherapy in advanced cirrhosis: a retrospective study. J Clin Gastroenterol 2011;45:643-50. 10.1097/MCG.0b013e318203dfb3 [DOI] [PubMed] [Google Scholar]


