Abstract
Background:
Fasciolosis is an important zoonotic disease affecting the productive performance of farm animals in Egypt.
Aim:
The aim of the present study was comparing the ovicidal effect of different extracts as an alcoholic (Methanolic and Ethanolic) and aqueous Moringa oleifera leaf extracts on Fasciola gigantica non-embryonated and developed eggs.
Materials and Methods:
Tested concentrations of extracts ranged from 12.5 to 800 mg/ml. Nitroxynil was used as reference drug with a dose of 100 mg/ml.
Results:
M. oleifera alcoholic and aqueous extracts showed a concentration-dependent ovicidal effect on F. gigantica non-embryonated and developed eggs. Based on LC50 values, water extract showed the highest ovicidal activity since it registered the lowest values of 2.6 mg/ml on non-embryonated eggs. Non-embryonated eggs were more susceptible to aqueous extract than developed eggs. On the other hand, the developed eggs were more susceptible to ethanolic extract than non-embryonated eggs even the lowest LC50 (12.38 mg/ml).
Conclusion:
M. oleifera leaf extracts especially aqueous extract could be a promising step in the field of controlling fascioliasis. Further, in vivo studies are needed to enlighten the therapeutic potential of M. oleifera extracts in treating F. gigantica infection.
Keywords: Fasciola gigantica, leaf extract, Moringa oleifera, nitroxynil, ovicidal activity
Introduction
Fasciolosis is considered one of the most important helminthic diseases affecting livestock in many countries around the world. It is commonly found in sheep and cattle [1]. Bovine fasciolosis usually had no visible clinical signs. However, if the cattle infection was chronic, it might cause weight gain loss, reduction in the milk yield, and fertility problems [2,3].
Many available chemotherapeutic anthelmintics had side effects on the host, and it is necessary to decrease the use of these drugs for parasitic control, not only for their resistance but also because of growing concerns about the adverse consequences on the ecosystem. Naturally produced plant anthelmintics offer an alternative that overcomes some of these effects and is both sustainable and environmentally acceptable [4]. Around the world, new plants having medicinal properties against parasites of ruminants had been explored, and they had shown good results [5]. Moringa oleifera is considered as one of the most useful trees for the reason that every part has many beneficial properties [6]. M. oleifera is commonly known as Drumstick and contains many active compounds such as alkaloids, flavonoids, saponins, tannins, and triterpenoids [7]. Extracts from this plant have several pharmacological effects such as anthelminthic [8-10], anti-inflammatory, antimicrobial, and antioxidant [11].
The aim of the current study was to compare the ovicidal effect of different extracts as an alcoholic (methanol and ethanol) and aqueous M. oleifera leaf extracts on Fasciola gigantica non-embryonated and developed eggs.
Materials and Methods
Ethical approval
F. gigantica eggs were recovered from gallbladders of cattle and buffaloes slaughtered in the government abattoir according to governmental regulations.
Collection of plant materials
Moringa leaves were collected from Pilbis, Sharqia Governorate, Egypt, kindly by Prof. Aboelfetoh M Abdalla, Moringa Unit, National Research Centre. The leaves were cleaned with tap water 3 times then by phosphate buffer (pH.7.4).
Preparation of extracts
Alcoholic extract
150 g of M. oleifera green leaves were separately extracted in 1500 ml of 80% ethanol (ethanolic extract) and 80% methanol (methanolic extract), then manually shaken for 30 min and allowed to stand with continuous shaking at a shaking water bath for 3 days. After that, solutions were filtered with sterile filter papers (Whatman No.1) into a clean conical flask and dried under reduced pressure in a rotary evaporator at a temperature below 50°C and stored at 4°C until use.
Aqueous extract
10 g of M. oleifera shade dried leaves were ground into powder and extracted by maceration in 1000 ml of double distilled water accompanied by stirring for 24 h in cold conditions 4°C then filtered with sterile filter papers (Whatman No.1) into a clean conical flask and stored at −20°C until use.
Drug of choice
Nitroxynil 25% (Devomor®) (Arabcomed for El-Nehesi Company, Egypt) and the dose was 100 mg/ml.
Collection of F. gigantica eggs
F. gigantica eggs were recovered from bile of the gallbladders of infected cattle and buffaloes slaughtered in Cairo local abattoir, Egypt. Gallbladders were washed and examined individually. Each gallbladder was evacuated separately in the 1 L cylinder, mixed with tap water, left to sediment and then the supernatant was decanted without disturbance of the sediment. This process was repeated 3-5 times. The clean F. gigantica eggs were collected after sedimentation and stored in distilled water to be used as fresh as possible.
Ovicidal effect of M. oleifera on F. gigantica eggs
Two experiments were involved in the present study; one was for non-embryonated and the other was for developed (morula stage) F. gigantica eggs. The alcoholic extracts concentrations were 12.5, 25, 50, 100, and 200 mg/ml, while in aqueous extract they were 100, 200, 400, and 800 mg/ml. Three replicates of every dilution were prepared in each treatment. Each replicate was contained 10 ml double distilled water and about 100 F. gigantica eggs and the selected extract concentration. Three replicates of 100 mg/ml nitroxynil drug were tested. Another group of eggs were incubated in distilled water and served as control group. The Petri dishes were incubated at 28°C in dark environment for 14 days. The numbers of hatched and non-developed eggs were counted. F. gigantica eggs and their developmental stages including the morula, eyespot stage or embryonated eggs and hatched eggs were identified as previously described [12].
The percentage of hatched and developed eggs for each treatment was calculated [13], and hatching ratio was calculated according to Canevari et al. [14].
Statistical analysis
Data of ovicidal effect of different treatment groups were analyzed for the means and standard deviations. The significance of the results was evaluated using independent sample t-test and analysis of variance and Duncan test using Statistical Package for the Social Sciences computer programs [15].
Results
The ovicidal effects of the different M. oleifera extract on the F. gigantica eggs are displayed in Tables-1 and 2. M. oleifera alcoholic and aqueous extracts, as well as nitroxynil, showed an ovicidal effect on Fasciola non-embryonated and developed eggs (Figures 1-6). The effect of these extracts on eggs was concentration dependent. There were no statistically significant differences between the ovicidal activity of M. oleifera methanolic and ethanolic extracts in non-embryonated eggs. Water extract at a concentration of 800 mg/ml had the same effect on eggs as 100 mg/ml methanolic and ethanolic extracts. In the developed eggs experiment, the highest concentrations of the three extracts were statistically the same.
Table-1.
Ovicidal effect of Moringa on Fasciola non-embryonated eggs.
| Treatment | Conc. (mg/ml) | Mean±SD | ||||
|---|---|---|---|---|---|---|
| Non-developed eggs (%) | Hatched eggs (%) | Hatching ratio | Ovicidal activity (%) | |||
| Moringa methanolic extract | 12.5 | 38.22±1.03h | 61.78±1.03b | 0.7±0.01b | 31.36±1.14h | |
| 25 | 67.68±1.49f | 32.32±1.49d | 0.37±0.02d | 64.09±1.65f | ||
| 50 | 79.49±2.91de | 20.51±2.91ef | 0.24±0.03e | 77.22±3.23de | ||
| 100 | 88.63±0.66b | 11.37±0.66h | 0.13±0.01gh | 87.36±0.73b | ||
| 200 | 98.98±0.01a | 1.02±0.01i | 0.01±0i | 98.87±0.01a | ||
| Moringa ethanolic extract | 12.5 | 42.55±0.77g | 57.45±0.77c | 0.64±0.01c | 36.17±0.85g | |
| 25 | 43.31±1.26g | 56.69±1.26c | 0.63±0.14c | 37.01±1.4g | ||
| 50 | 79.76±0.5de | 20.24±0.5ef | 0.22±0.01ef | 77.51±0.56de | ||
| 100 | 89.25±3.06b | 10.75±3.06h | 0.12±0.03jk | 88.06±3.4b | ||
| 200 | 98.33±1.53a | 1.67±1.53i | 0.02±0.01i | 98.15±1.7a | ||
| Moringa water extract | 100 | 85.74±1.06c | 14.26±1.06g | 0.2±0.02f | 84.16±1.18c | |
| 200 | 90±2b | 10±2h | 0.16±0.01g | 88.89±2.2b | ||
| 400 | 77.42±1.06e | 22.58±1.06e | 0.11±0.02h | 74.91±1.18e | ||
| 800 | 91.13±0.78b | 8.87±0.78h | 0.1±0.01h | 90.14±0.87b | ||
| Control (Dist. water) | - | 10±0.91i | 89.99±0.95a | 1±0a | 0±0i | |
| Nitroxynil | 100 | 81.84±1.57d | 18.16±1.57f | 0.25±0.02e | 79.82±1.74d | |
| F value | 859.94 | 859.94 | 744.23 | 881.83 | ||
| p | p<0.001 | p<0.001 | p<0.001 | p<0.001 | ||
All data expressed as mean±SD. Means followed by different letters indicated significance. SD=Standard deviation
Table-2.
Ovicidal effect of Moringa on Fasciola developed eggs.
| Treatment | Conc. (mg/ml) | Mean±SD | ||||
|---|---|---|---|---|---|---|
| Nondeveloped eggs (%) | Hatched eggs (%) | Hatching ratio | Ovicidal activity (%) | |||
| Moringa methanolic extract | 12.5 | 46.48±1.38h | 53.91±1.01b | 0.61±0.02b | 39.06±1.14h | |
| 25 | 59.96±1.65g | 39.73±1.43c | 0.46±0.02c | 55.09±1.61g | ||
| 50 | 72.02±2.11e | 27.18±0.77e | 0.32±0.02e | 69.27±0.87e | ||
| 100 | 88.58±2.01b | 11.87±1.69h | 0.13±0.02i | 86.59±1.91b | ||
| 200 | 94.68±2.1a | 5.10±2.02i | 0.06±0.02j | 94.24±2.29a | ||
| Moringa ethanolic extract | 12.5 | 47.06±2.67h | 52.94±2.67b | 0.60±0.03b | 40.16±3.02h | |
| 25 | 63.99±1.48f | 36.01±1.48d | 0.41±0.02d | 59.3±1.67f | ||
| 50 | 80.07±0.95d | 19.93±0.95f | 0.23±0.01g | 77.47±1.07d | ||
| 100 | 86.33±0.72bc | 13.67±0.72gh | 0.16±0.01hi | 84.54±0.81bc | ||
| 200 | 94.10±2.35a | 5.90±2.35i | 0.07±0.03j | 93.33±2.65a | ||
| Moringa water extract | 100 | 64.4±1.68f | 35.6±1.68d | 0.39±0.02d | 62.31±1.78f | |
| 200 | 79.6±1.22d | 20.4±1.22f | 0.22±0.01g | 78.4±1.29d | ||
| 400 | 84.09±2.01c | 15.91±2.01g | 0.17±0.02h | 83.16±2.13c | ||
| 800 | 95.37±1.46a | 4.63±1.46i | 0.05±02j | 95.09±1.54a | ||
| Control (Dist. water) | - | 11.54±1.54i | 88.46±1.68a | 1±0a | 0±0i | |
| Nitroxynil | 100 | 75.03±2.4e | 24.97±2.4e | 0.28±0.03f | 71.77±2.72e | |
| F value | 454.07 | 522.79 | 473.97 | 577.01 | ||
| p | p<0.001 | p<0.001 | p<0.001 | p<0.001 | ||
All data expressed as mean±SD. Means followed by different letters indicated significance. SD=Standard deviation
Figure-1.
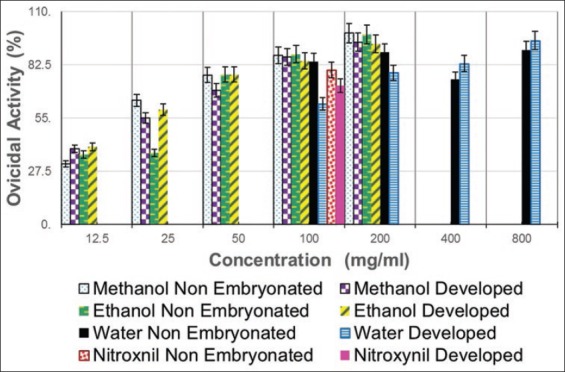
Ovicidal activities of Moringa extracts and nitroxynil on non-embryonated and developed Fasciola eggs.
Figure-2.
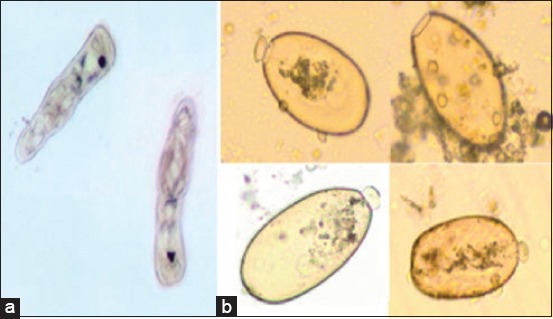
Control untreated Fasciola gigantica eggs. (a): Hatched miracidia with eye spots, (b): Hatched eggs (empty eggs with opened operculum) (40×).
Figure-3.
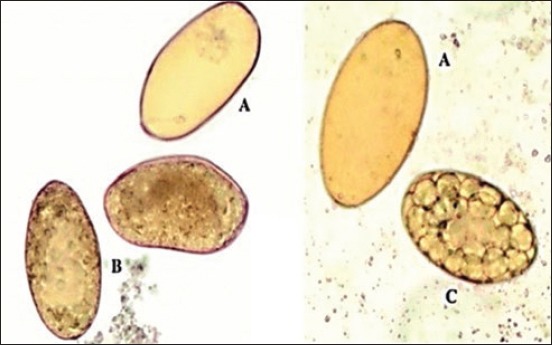
Moringa treated Fasciola eggs (methanol extract) at highest concentration 200 mg/ml: (A) Empty Fasciola eggs with a closed operculum, (B) dead eggs with lysed embryo, (C) eggs of early development (40×).
Figure-4.
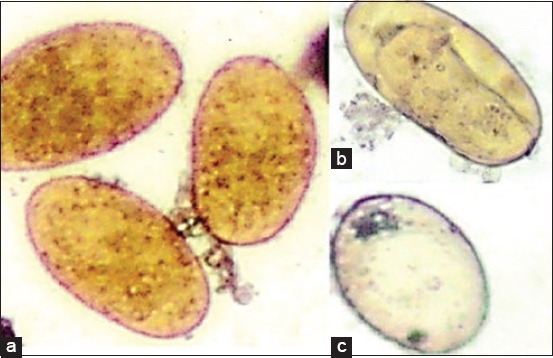
Moringa treated Fasciola eggs (Ethanol extract) at highest concentration 200 mg/ml: (a) Degenerated eggs, (b) dead miracidium inside the egg, (c) empty Fasciola egg with a closed operculum (40×).
Figure-5.
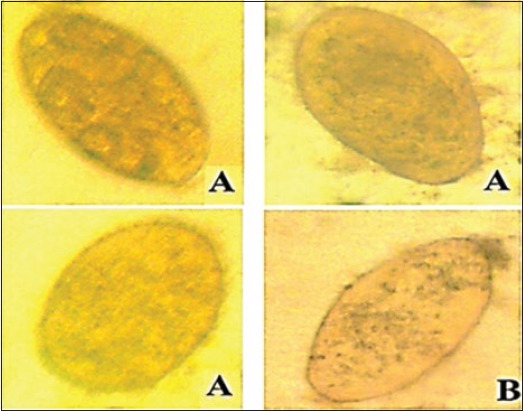
Moringa treated Fasciola eggs (water extract) at highest concentration 800 mg/ml: (A) Degenerated eggs and (B) empty Fasciola egg with a closed operculum (40×).
Figure-6.
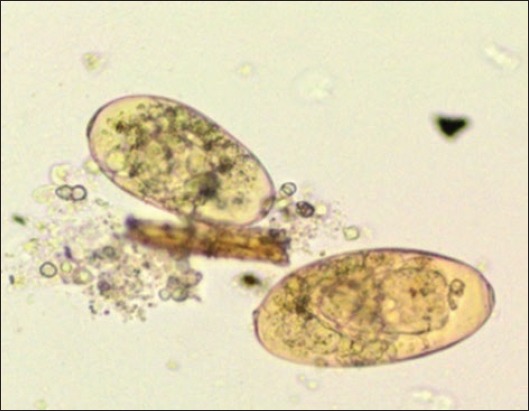
Nitroxynil treated Fasciola eggs at concentration 100 mg/ml: Degenerated eggs (40×).
Regarding the comparing the ovicidal activity of the three extracts on the eggs with independent sample t-test, significant differences were found between the 2 stages of eggs development at almost all concentrations of methanolic and aqueous extracts. Almost all concentrations of ethanolic extract had the same effect on non-embryonated and developed eggs (Table-3).
Table-3.
Ovicidal activity of Moringa extracted and nitroxynil in non-embryonated and developed Fasciola eggs.
| Treatment | Conc. (mg/ml) | Non-embryonated Fasciola eggs | Developed Fasciola eggs | T | p |
|---|---|---|---|---|---|
| Moringa methanolic extract | 12.5 | 31.36±1.14 | 39.06±1.14 | 8.26 | 0.001** |
| 25 | 64.09±1.65 | 55.09±1.61 | 6.74 | 0.003** | |
| 50 | 77.22±3.23 | 69.27±0.87 | 4.11 | 0.02* | |
| 100 | 87.36±0.73 | 86.59±1.91 | 0.66 | 0.5NS | |
| 200 | 98.87±0.01 | 94.24±2.29 | 3.51 | 0.03* | |
| Moringa ethanolic extract | 12.5 | 36.17±0.85 | 40.16±3.02 | 2.2 | 0.09NS |
| 25 | 37.01±1.4 | 59.3±1.67 | 17.73 | 0.000*** | |
| 50 | 77.51±0.56 | 77.47±1.07 | 0.05 | 0.96NS | |
| 100 | 88.06±3.4 | 84.54±0.81 | 1.74 | 0.16NS | |
| 200 | 98.15±1.7 | 93.33±2.65 | 2.65 | 0.06NS | |
| Moringa water extract | 100 | 84.16±1.18 | 62.31±1.78 | 17.77 | 0.000*** |
| 200 | 88.89±2.2 | 78.4±1.29 | 7.07 | 0.002** | |
| 400 | 74.91±1.18 | 83.16±2.13 | 5.87 | 0.004** | |
| 800 | 90.14±0.87 | 95.09±1.54 | 4.837 | 0.008** | |
| Control (Dist. water) | - | 0±0 | 0±0 | 1.37 | 0.24NS |
| Nitroxynil | 100 | 79.82±1.74 | 71.77±2.72 | 4.32 | 0.01* |
All data expressed as mean±SD.
Significant differences at p<0.05.
Significant differences at p<0.01.
Significant differences at p<0.001.
NSNon-significant. Means followed by different letters indicated significance. SD=Standard deviation
LC50 of the different concentrations was calculated as shown in Table-4. LC50 was higher in non-embryonated eggs than developed eggs for methanolic and ethanolic extracts. Water extract exhibited a different effect as LC50 of aqueous extract on developed was higher than that of non-embryonated eggs.
Table-4.
LC50 of different Moringa extracts on Fasciola nonembryonated and developed eggs
| Fasciola egg | Moringa extract | LC50 | |||
|---|---|---|---|---|---|
| Conc. (mg/ml) | Lower limit | Upper limit | |||
| Nonembryonated eggs | Methanol | 19.98 | 12.42 | 26.42 | |
| Ethanol | 24.39 | 10.7 | 36.93 | ||
| Water | 2.6 | 0.03 | 12.31 | ||
| Developed eggs | Methanol | 15.85 | 13.21 | 18.45 | |
| Ethanol | 12.38 | 4.15 | 16.36 | ||
| Water | 60.69 | 44.12 | 76.46 | ||
Discussion
In the current study, M. oleifera leaf extracts presented concentration-dependent activities against the two developmental stages of F. gigantica eggs tested. These results are similar to those obtained by Pessoa et al. [16] against Haemonchus contortus by the essential oil of Ocimum gratissimum. Furthermore, they confirmed the results obtained previously on rhabditiform larvae of Ancylostoma caninum by Poné et al. [17] and on eggs of the nematode A. caninum with the extracts of the shrub Canthium mannii by Poné et al. [18].
Alcoholic and aqueous extracts of M. oleifera represented high anthelmintic activity on eggs. In addition, all extracts rendered most of non-embryonated F. gigantica eggs undeveloped suggesting that the bioactive compounds were lethal to the blastomeres. Furthermore, Rastogi et al. [8] found that the ethanolic extract of M. oleifera showed strong anthelmintic activity at 25, 50, and 100 mg/ml against Indian earthworm. On the contrary, M. oleifera did not give satisfactory in vitro anthelmintic results on Ascaris suum with Peter and Deogracious [19] with a median effective dose more than 50 mg/ml in the initial screening.
The ovicidal activities observed in the present study using different extracts might be attributed to the presence of saponins, steroids, carbohydrates, alkaloids, tannins, and flavonoids which were reported in M. oleifera leaves [20,21]. Previously, it was suggested that tannins and saponins possibly penetrated the egg affecting the morula [22] and stop hatching [23] of H. contortus eggs.
In the current study, there were no statistically significant differences between ovicidal activity of M. oleifera methanolic and ethanolic extracts in non-embryonated and developed eggs. The probable reason for the minor differences between aqueous and alcoholic extracts could be due to variation in solubility of the active compounds in the solvent. Infused aqueous extract and ethanolic extract of M. oleifera tested by Tayo et al. [24] on H. contortus, in vitro, presented comparable activity on eggs. The observed similar activity of ethanolic extract and infused aqueous extract of M. oleifera leaves on eggs could be due to the presence of similar or related chemicals having ovicidal properties in approximately equivalent proportions. On the contrary, Poné et al. [18] found that the ethanol extract was more potent on the eggs when compared to hot and cold water extracts.
Based on LC50 values, water extract presented the highest ovicidal activity since it registered the lowest values of 2.6 mg/ml on immature non-embryonated eggs suggesting that non-embryonated eggs were more susceptible to aqueous extract than developed eggs. On the other hand, developing eggs were more susceptible to ethanolic extract than non-embryonated eggs as ethanolic extract had the lowest LC50 (12.38 mg/ml). These results agreed with Tayo et al. [24] who found that ethanolic extracts of M. oleifera leave presented the highest activity on late stages of development of H. contortus eggs since it registered the lowest values of LC50. They disagreed with Poné et al. [18] found that LC50 value of the ethanol extract of C. mannii was relatively low, indicating that this extract is more active on non-embryonated eggs than aqueous extracts. This conflict might be due to they used H. contortus eggs, and in the current study, F. gigantica eggs were used. Although not quite exact on how M. oleifera inhibit egg embryonation, Sreelatha et al. [25] explained that M. oleifera leaf extract could induce cellular apoptosis, morphological change and DNA fragmentation in a type of human cancer cell.
Conclusion
The current study introduced M. oleifera leaf aqueous extract which offered a promising ovicidal effect on F. gigantica eggs. The future scope involves the need of in vivo study to enlighten the therapeutic potential of M. oleifera extracts in treating F. gigantica infection. Furthermore, isolation of phytoconstituents responsible for these activities is required.
Authors’ Contributions
This study was designed and supervised by AGH. While, SEH, EEE and DA carried out extracts preparation. AMM and NIT collected the samples. KNA, SEH, EEE and DA examined gallbladders. KNA, SEH, DA and EEE carried out the application of plant extracts on eggs. DA analyzed and interpreted the data. All authors have prepared, read and approved the final manuscript.
Acknowledgments
This work was financially supported by the project number 11020201 entitled: “Evaluation of antimicrobial effect of propolis and Moringa on Fasciola gigantica and Clostridium oedematiens (Clostridium novyi) type B” which is funded by a grant from National Research Centre (NRC), Egypt.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Shokier K.M, Aboelhadid S.M, Waleed M.A. Efficacy of five anthelmintics against a naturalFasciolaspecies infection in cattle. BJBAS. 2013;2:4–5. [Google Scholar]
- 2.Schweizer G, Braun U, Deplazes P, Torgerson P.R. Estimating the financial losses due to bovine fasciolosis in Switzerland. Vet. Rec. 2005;157:188–193. doi: 10.1136/vr.157.7.188. [DOI] [PubMed] [Google Scholar]
- 3.Charlier J, Duchateau L, Claerebout E, Williams D, Vercruysse J. Associations between anti-Fasciola hepaticaantibody levels in bulk-tank milk samples and production parameters in dairy herds. Prev. Vet. Med. 2007;78:57–66. doi: 10.1016/j.prevetmed.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Veerakumari L. Botanical anthelmintics. Asian J. Sci. Tech. 2015;6:1881–1894. [Google Scholar]
- 5.Bauri R.K, Tigga M.N, Kullu S.S. A review on use of medicinal plants to control parasites. Ind. J. Natl. Prod. Resour. 2015;6:268–277. [Google Scholar]
- 6.Devendra B.N, Srinivas N, Talluri P.S, Latha P.S. Antimicrobial Activity ofMoringa oleiferaLam., leaf extract, against selected bacterial and fungal strains. Int. J. Pharm. BioSci. 2011;2:13–18. [Google Scholar]
- 7.Nilani P, Pinaka M.K, Duraisamy B, Dhamodaran P, Jeyaprakash M.R. Anthelmintic activity ofMoringa Oleiferaseed oil - Validation of traditional use. J. Adv. Sci. Res. 2012;3:65–66. [Google Scholar]
- 8.Rastogi T, Bhutda V, Moon K, Aswar P.B, Khadabadi S.S. Comparative studies on anthelmintic activity ofMoringa oleiferaandVitex negundo. Asian J. Res. Chem. 2009;2:181–182. [Google Scholar]
- 9.Konmy B.B.S, Olounladé P.A, Doko-allou S, Azando E.B.V, Hounzangbé-Adoté S.E.G. A review on phytochemistry and pharmacology ofMoringa oleiferaleaves (Moringaceae) J. Pharm. Phytochem. 2016;5:325–330. [Google Scholar]
- 10.Martínez-González C.L, Martínez L, Martínez-Ortiz E.J, González-Trujano M.E, Déciga-Campos M, Ventura-Martínez R, Díaz-Reval I. Moringa oleiferaa species with potential analgesic and anti-inflammatory activities. Biomed. Pharmacother. 2017;87:482–488. doi: 10.1016/j.biopha.2016.12.107. [DOI] [PubMed] [Google Scholar]
- 11.Mbikay M. Therapeutic postential ofMoringa oleiferaleaves in chronic hyper-glycemicemia and dyslipidemia:A review. Front. Pharmacol. 2012;3:1–37. doi: 10.3389/fphar.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fairweather I, McShane D, Shaw L, Ellison S.E, O'Hagan N.T, York E.A, Trudgett A, Brennan G.P. Development of an egg hatch assay for the diagnosis of triclabendazole resistance inFasciola hepatica:Proof of concept. Vet. Parasitol. 2012;183:249–259. doi: 10.1016/j.vetpar.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Arafa W.A, Shokeir K.M, Khateib A.M. Comparing anin vivoegg reduction test andin vitroegg hatching assay for different anthelmintics againstFasciolaspecies, in cattle. Vet. Parasitol. 2015;214:152–158. doi: 10.1016/j.vetpar.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Canevari J, Ceballos L, Sanabria R, Romero J. Testing albendazole resistance inFasciola hepatica:Validation of an egg hatch test with isolates from South America and the United Kingdom. J. Helminthol. 2014;88:286–292. doi: 10.1017/S0022149X13000163. [DOI] [PubMed] [Google Scholar]
- 15.SPSS 15.0. Statistical Package for Social Science, SPSS for Windows Evaluation Version. Standard Version, Copyright SPSS Inc., 1989-2006. Buckingham. 2006 [Google Scholar]
- 16.Pessoa L.M, Movais S.M, Bevilaqaliua C.M.L, Luciano J.H.S. Anthelmintic activity of essential oil ofOcimum gratissimumLinn, eugenol againstHaemonchus contortus. Vet. Parasitol. 2002;109:59–63. doi: 10.1016/s0304-4017(02)00253-4. [DOI] [PubMed] [Google Scholar]
- 17.Poné W.J, Mpoame M, Bilong B.C.F, Kerboeuf D. Etude comparein vitrode l'activiténématodicide de l'extrait éthanolique de la poudre d'écorce deCanthium mannii(Rubiaceae) et duMébendazole. Rev. Méd. Vét. 2005;156:633–636. [Google Scholar]
- 18.Poné W.J, Bilong B.C.F, Mpoame M, Ngwa C.F, Coles C.G. In vitroactivity of ethanolic, cold and hot water extracts of the stem bark ofCanthium mannii(Rubiaceae) stem onAncylostoma caninumeggs. East Cent. Afr. J. Pharm. Sci. 2006;9:14–18. [Google Scholar]
- 19.Peter W, Deogracious O. Thein-vitroascaricidal activity of selected indigenous medicinal plants used in ethno veterinary practices in Uganda. Afr. J. Trad. Cam. 2006;3:94–103. [Google Scholar]
- 20.Fatima T, Sajid M.S, Hassan M.J.U, Siddique R.M, Iqbal Z. Phytomedicinal value ofMoringa oleiferawith special reference to antiparasitics. Pak. J. Agric. Sci. 2014;51:251–262. [Google Scholar]
- 21.Ali S.A, Mohamed A.H, Gameel A.A, Hassan T. Protective Effect ofMoringa OleiferaLeaves Aqueous Extract against Carbon Tetrachloride Induced Hepatotoxicity in Rats. 14th ed. Assiut, Egypt: Science Congress Faculty of Veterinary Medicine; 2010. pp. 327–336. [Google Scholar]
- 22.Cabardo D.E, Portugaliza H.P. Anthelmintic activity ofMoringa oleiferaseed aqueous and ethanolic extracts againstHaemonchus contortuseggs and third stage larvae. Int. J. Vet. Sci. Med. 2017;5:30–34. doi: 10.1016/j.ijvsm.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vargas-Magaña J.J, Torres-Acosta J.F.J, Aguilar-Caballero A.J, Sandoval-Castro C.A, Hoste H, Chan-Pérez J.I. Anthelmintic activity of acetone–water extracts againstHaemonchus contortuseggs:Interactions between tannins and other plant secondary compounds. Vet. Parasitol. 2014;206:322–327. doi: 10.1016/j.vetpar.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Tayo G.M, Poné J.W, Komtangi M.C, Yondo J, Ngangout A.M, Mbida M. Anthelminthic activity ofMoringa oleiferaleaf extracts evaluatedin vitroon four developmental stages ofHaemonchus contortusfrom goats. Am. J. Plant Sci. 2014;5:1702–1710. [Google Scholar]
- 25.Sreelatha S, Jeyachitra A, Padma P.R. Antiproliferation and induction of apoptosis byMoringa oleiferaleaf extract on human cancer cells. Food Chem. Toxicol. 2011;49:1270–1275. doi: 10.1016/j.fct.2011.03.006. [DOI] [PubMed] [Google Scholar]


