ABSTRACT
Staphylococcus aureus persistently colonizes the nasopharynx in humans, which increases the risk for invasive diseases, such as skin infection and bacteremia. Nasal colonization triggers IgG responses against staphylococcal surface antigens; however, these antibodies cannot prevent subsequent colonization or disease. Here, we describe S. aureus WU1, a multilocus sequence type 88 (ST88) isolate that persistently colonizes the nasopharynx in mice. We report that staphylococcal protein A (SpA) is required for persistence of S. aureus WU1 in the nasopharynx. Compared to animals colonized by wild-type S. aureus, mice colonized with the Δspa variant mount increased IgG responses against staphylococcal colonization determinants. Immunization of mice with a nontoxigenic SpA variant, which cannot cross-link B cell receptors and divert antibody responses, elicits protein A-neutralizing antibodies that promote IgG responses against colonizing S. aureus and diminish pathogen persistence.
IMPORTANCE Staphylococcus aureus persistently colonizes the nasopharynx in about one-third of the human population, thereby promoting community- and hospital-acquired infections. Antibiotics are currently used for decolonization of individuals at increased risk of infection. However, the efficacy of antibiotics is limited by recolonization and selection for drug-resistant strains. Here, we propose a model of how staphylococcal protein A (SpA), a B cell superantigen, modifies host immune responses during colonization to support continued persistence of S. aureus in the nasopharynx. We show that this mechanism can be thwarted by vaccine-induced anti-SpA antibodies that promote IgG responses against staphylococcal antigens and diminish colonization.
KEYWORDS: Staphylococcus aureus, colonization, immune response, immunity, staphylococcal protein A
INTRODUCTION
Staphylococcus aureus is a frequent cause of community- and hospital-acquired diseases, including skin and soft tissue infections, pneumonia, bacteremia, and endocarditis (1). Between 20 and 41% of the human population are persistently colonized by S. aureus, while others serve as intermittent carriers of the pathogen (2). S. aureus is predominately located in the anterior nasal vestibule and is also isolated from the oropharynx and gastrointestinal tract (3–5). Colonization with S. aureus constitutes a major risk for community- and hospital-acquired infections (6, 7). Antibiotic decolonization serves the dual purposes of reducing the risk of infection in individual carriers and preventing the spread of S. aureus, particularly in hospital settings (8). Mupirocin treatment is currently used for decolonization; however, its long-term success is low due to recolonization and selection for mupirocin-resistant strains (9).
S. aureus colonization occurs in the first weeks of life, as staphylococci can be readily isolated from the nasopharynx and perineum in 24 to 46% of infants (10). Colonization is associated with increases in serum IgG titers against secreted staphylococcal antigens, including sortase-anchored surface proteins and secreted toxins (11–13). Of note, S. aureus colonization, as well as invasive disease, increases the relative abundance of pathogen-specific IgG4 antibody responses compared to those of IgG1 subclass antibodies (12). However, serum IgG responses to S. aureus colonization or infection are not considered protective against either further colonization or subsequent invasive disease (7, 14, 15). No FDA-licensed vaccine capable of preventing S. aureus colonization or invasive disease is currently available (16).
Earlier work sought to identify S. aureus genes required for nasal colonization, using bacterial adherence to human desquamated nasal epithelial cells and in vivo colonization of cotton rats as models (17, 18). Another model system, nasal colonization of mice with human clinical isolates, typically requires prior antibiotic treatment to deplete the resident microbiota and to provide selection for colonization with antibiotic-resistant strains (19). These in vitro and in vivo model systems identified several surface components that are necessary for S. aureus colonization (20). Specifically, clumping factor B (ClfB) promotes staphylococcal adherence by binding to loricrin and cytokeratin 10 in nasal epithelia (21). Compared with wild-type S. aureus, an isogenic clfB mutant was cleared more rapidly from the nasal epithelia of human volunteers (7). Serine-aspartate repeat surface proteins C (SdrC) and D (SdrD), as well as iron-regulated surface determinant A (IsdA), also contribute to staphylococcal adherence to human nasal epithelial cells (17, 22). IsdA contributes to iron scavenging from host hemoproteins and also binds lactoferrin, which inhibits the antistaphylococcal activity of lactoferrin in human nasal secretions (23, 24). S. aureus surface protein G (SasG) mediates zinc-dependent adhesion between bacterial cells during biofilm formation and adherence to nasal tissue (25, 26). Finally, S. aureus synthesizes cell wall-linked wall teichoic acid (WTA), a polymer of ribitol-phosphate, with esterified d-alanyl (d-Ala) and α- and/or β-linked N-acetylglucosamine (GlcNAc) (20). d-Ala and GlcNAc modifications of WTA also contribute to staphylococcal adherence by binding the type F scavenger receptor (SREC-I) of human nasal epithelia and supporting nasal colonization in cotton rats (18, 27, 28).
The role of staphylococcal protein A (SpA) during S. aureus nasal colonization has been enigmatic (29). In contrast to many toxin and capsular polysaccharide genes and several other surface protein genes, S. aureus expresses spa during colonization of both humans and cotton rats (30, 31). Although the tandem-repeat structure of the spa gene promotes high-frequency recombination, human colonization selects for spa alleles whose products maintain five immunoglobulin binding domains (IgBDs), which endows staphylococci with potent B cell superantigen activity (32, 33). When analyzed in human volunteers who had been cleared of nasal carriage via mupirocin treatment, S. aureus spa expression was not required for bacterial adherence to human nasal tissue and for initial colonization, i.e., for a 10-day period following inoculation (34). In contrast, a human methicillin-resistant S. aureus (MRSA) multilocus sequence type 239 (ST239) isolate was reported to require spa expression for nasal adherence and 3-day colonization of mice that had been pretreated with ampicillin (35).
S. aureus JSNZ is a member of ST88, which is rare in human populations (36). Strain JSNZ was isolated from an outbreak of preputial gland abscesses among male C57BL/6 mice (36). Unlike human clinical isolates, S. aureus JSNZ persistently colonizes the nasopharynxes of mice without prior antibiotic treatment (36, 37). Here, we report the isolation of S. aureus WU1, another ST88/clonal complex 88 (CC88) isolate causing preputial gland abscess lesions in male mice. Similar to strain JSNZ, S. aureus WU1 persistently colonizes the nasopharynxes of mice and promotes serum IgG responses against staphylococcal surface molecules. Compared with wild-type S. aureus WU1, the Δspa mutant displays a persistence defect during colonization and elicits increased IgG responses against staphylococcal surface molecules. Immunization of mice with purified SpAKKAA, a protein A variant that cannot bind IgG Fcγ or cross-link VH3 idiotype B cell receptors, generates SpA-neutralizing antibodies that promote increased pathogen-specific IgG responses and decolonization of S. aureus ST88 isolates.
RESULTS
S. aureus WU1.
An outbreak of preputial gland infections of male C57BL/6 mice was observed in an animal breeding colony. Samples were collected from preputial gland adenitis (PGA) and from the nasopharynxes of male and female C75BL/6 mice and analyzed by growth on mannitol-salt agar (MSA [BBL; Becton Dickinson]) and Baird-Parker agar (BPA [Difco; Becton Dickinson]). Multilocus sequence typing (MLST) and spa genotyping revealed that the animals had been colonized with S. aureus ST88 spa genotype t186, which was also responsible for PGA in male mice. S. aureus CC88 with spa genotype t186 has been reported before as stably colonizing isolates from laboratory mice in the United States (37). Other spa genotypes include t325, t448, t690, t755, t786, t2085, t2815, t5562, t11285, and t12341 (37). The New Zealand JSNZ isolate carries the distinct spa genotype t729 (37). Nonetheless, both S. aureus JSNZ and WU1 share the type 8 capsular polysaccharide genes and lack the mecA gene, as well as mobile genetic element (MGE)-encoded T cell superantigens (37). Further, the hlb-converting phage that expresses human-specific immune evasion cluster 1 (IEC1) genes sak (staphylokinase), chp (CHIPS [chemotaxis-inhibitory protein of S. aureus]), and scn (SCIN-A [staphylococcal complement inhibitor A]) is absent in the genome of WU1, resulting in an intact β-hemolysin-encoding gene (hlb) (38). Of note, the WU1-encoded IEC2 carries the scn homologue scb or scc (SCIN-B/SCIN-C), along with hla (α-hemolysin) and ssl12-14 (staphylococcal superantigen-like 12-14) (39). Unlike those of other CC88 isolates that stably colonize mice (37), the genome of WU1 harbors the blaZ gene. When analyzed for genes encoding sortase-anchored surface proteins, we observed that S. aureus WU1 carries genes for determinants previously associated with nasal colonization, including ClfB, IsdA, SdrC, SdrD, and SasG (Table 1).
TABLE 1.
Conservation of protein products of select open reading frames in the genomes of S. aureus WU1, JSNZ, and Newman
| Protein | Amino acid identity (%) to WU1 gene product |
|
|---|---|---|
| JSNZ | Newman | |
| SpA | 99 | 98 |
| ClfA | 100 | 93 |
| ClfB | 100 | 96 |
| FnBPA | 100 | 82 |
| FnBPB | 87 | 87 |
| IsdA | 100 | 100 |
| IsdB | 99 | 98 |
| SdrC | 100 | 100 |
| SdrD | 95 | 95 |
| SdrE | 100 | 98 |
| EsxA | 100 | 100 |
| EsxB | 100 | 100 |
| SasA | 100 | 99 |
| SasD | 100 | 99 |
| SasF | 100 | 98 |
| SasI | 99 | 100 |
| SasG | 100 | 69 |
| SasK | 100 | 93a |
| Coa | 98 | 98 |
| vWbp | 100 | 71 |
| Hla | 100 | 99 |
| SCIN | 100 | 45b |
| Eap | 100 | 99 |
| Efb | 100 | 99 |
| Ebh | 99 | 98 |
| TarS | 100 | 98 |
Compared to S. aureus strain 04-02981.
Compared to S. aureus strain USA300.
S. aureus abscess formation has been linked to determinants of bacterial agglutination with fibrin (40, 41). Agglutination requires two S. aureus-secreted products that activate host prothrombin to convert fibrinogen into fibrin: coagulase (Coa) and von Willebrand factor binding protein (vWbp) (40). Clumping factor A (ClfA) binds fibrinogen and coats staphylococci with coagulase-generated fibrin fibrils, thereby interfering with S. aureus uptake and killing by host phagocytes (41, 42). The clfA genes in S. aureus WU1 and JSNZ are identical yet display allele-specific differences from clfA of S. aureus Newman (Table 1), a CC8 human clinical isolate that is used routinely for laboratory challenge experiments with mice (43). The observed differences in clfA, however, are clade specific, as they can be found in CC88 strains isolated either from human or from murine hosts (data not shown). The coa gene products of S. aureus WU1, JSNZ, and Newman are virtually identical (Table 1). In contrast, the product of the vwb genes of S. aureus WU1 and JSNZ differ significantly from that of S. aureus Newman, with the greatest sequence variation in the prothrombin-binding D1 and D2 domains (Fig. 1A). vWbp from WU1 and JSNZ was not recognized by polyclonal antibodies raised against Newman vWbp (Fig. 1B). Secreted vWbp proteins from the two CC88 strains could be recognized by a serum that had been raised against the conserved C-terminal domain of vWbp from strain USA300 (Fig. 1C). In contrast to S. aureus Newman, which secretes large amounts of Coa and rapidly agglutinates human and mouse plasma, S. aureus WU1 and JSNZ secrete less Coa and agglutinate mouse plasma more readily than human plasma (Fig. 1B, D, and E). The coagulase activity of S. aureus Newman is dependent on coa and vwb expression, as the corresponding Δcoa, Δvwb, and Δcoa Δvwb mutants displayed agglutination defects in mouse and human plasma (Fig. 1D and E). Taken together, these data suggest that the ST88 alleles of the vwb genes in S. aureus WU1 and JSNZ may promote efficient prothrombin-mediated coagulation and fibrin agglutination in mouse plasma, which may support the pathogenesis of invasive diseases, such as PGA.
FIG 1.
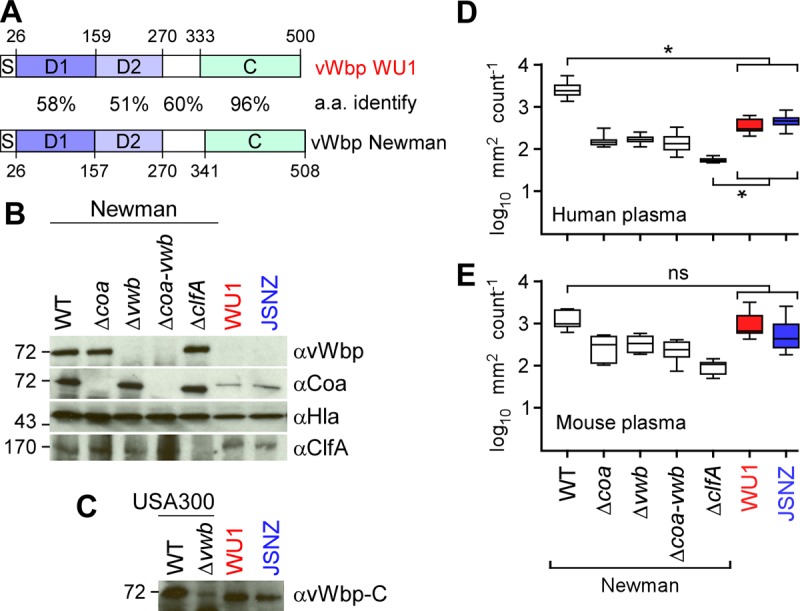
S. aureus ST88 isolate WU1, a mouse pathogen. (A) Domain structure and sequence homology of the vwb gene products from S. aureus WU1 and S. aureus Newman, a human clinical isolate. The percent amino acid (a.a.) identity of vWbp with its signal peptide (S), D1 and D2 domains (responsible for binding and activation of host prothrombin), linker (white box), and C-terminal fibrinogen binding domain (C) are displayed. (B) Immunoblots of S. aureus whole culture samples of strain Newman (wild type [WT]), as well as its Δcoa, Δvwb, Δcoa-vwb, and ΔclfA variants, WU1, and JSNZ, were analyzed for the production of vWbp (αvWbp), Coa (αCoa), Hla (αHla), and ClfA (αClfA). Sizes are given on the left in kilodaltons. (C) Polyclonal antibodies against the vWbp C domain identify the vWbp allelic variants from strains JSNZ and WU1, as well as vWbp from strain USA300 LAC. (D and E) Agglutination of Syto-9-stained S. aureus strains in human (D) or mouse (E) plasma was measured as the average sizes and standard errors of the mean of clumped bacteria in 12 microscope fields of view, and statistical significance was assessed in pairwise comparisons with the WT using two-way ANOVA with Sidak multiple-comparison tests. *, P < 0.05; ns, not significant.
S. aureus WU1 persistently colonizes the nasopharynx in mice.
To analyze S. aureus WU1 for its ability to colonize mice, cohorts (n = 10) of female C57BL/6 animals were analyzed by spreading pharyngeal swabs and fecal material on BPA. Naive mice lacking bacterial growth on BPA were anesthetized and inoculated by pipetting 10-μl suspensions of 1 × 108 CFU S. aureus WU1 in phosphate-buffered saline (PBS) into the right nostril. The animals were analyzed for colonization by swabbing the oropharynx at weekly intervals, i.e., 7, 14, 21, 28, 35, and 42 days following inoculation. The swabs were spread on BPA, incubated for colony formation, and enumerated (Fig. 2A). Even without prior antibiotic treatment or antibiotic selection, S. aureus WU1 colonized experimental animals with a load ranging from 0.8 to 3.1 log10 CFU per swab over 42 days (Fig. 2A). To validate persistent colonization with S. aureus WU1, colonies obtained after 42 days were analyzed by MLST and spa genotyping. The data showed that the mice were still colonized with ST88 spa t186, indicating that S. aureus WU1 persistently colonizes the nasopharynxes of C57BL/6 mice. As a control, mock (PBS) inoculation of cohorts of C57BL/6 animals in separate cages that were maintained in the same animal facility room and the same cage racks as S. aureus WU1-colonized animals did not lead to staphylococcal colonization of the nasopharynx (Fig. 2A). Day 42 stool samples from the mice were homogenized in PBS and plated on MSA for CFU enumeration (Fig. 2B). Stool samples from S. aureus WU1-colonized mice harbored 5.1 to 7.3 log10 CFU g−1 feces, indicating that the gastrointestinal (GI) tract was also colonized with S. aureus strain WU1. As a control, mock (PBS)-inoculated mice did not harbor S. aureus in their stool samples (Fig. 2B).
FIG 2.
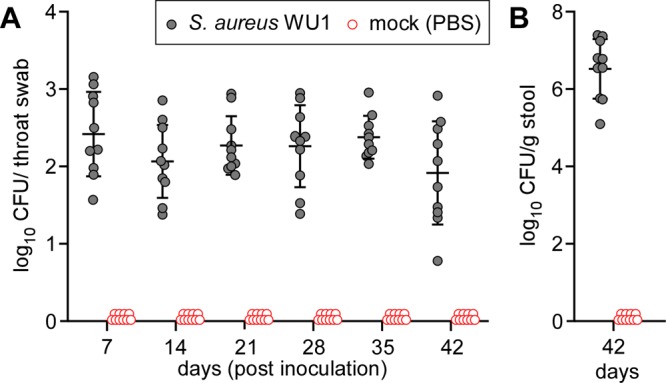
S. aureus WU1 persistently colonizes the nasopharynxes of C57BL/6 mice. Cohorts of C57BL/6 mice (n = 10) were inoculated intranasally with 1 × 108 CFU of the indicated S. aureus WU1 or PBS control. (A) Mice were swabbed in the throat weekly to enumerate the bacterial load. Each dot indicates the number of CFU in one mouse. (B) Stool samples were collected on day 42 following inoculation to enumerate the bacterial load. Each dot indicates the number of CFU per milliliter per gram of stool. The median and standard deviation for each group of animals on a given day are indicated by the horizontal lines and error bars.
S. aureus WU1 colonization triggers serum IgG response in mice.
Earlier work generated the S. aureus antigen matrix, which is comprised of 25 conserved secreted proteins. Each of the 25 recombinant affinity-tagged proteins was purified and immobilized on a membrane filter (44). To measure host immune responses during colonization, naive or S. aureus WU1-colonized animals were bled 15 days after inoculation, and serum IgG responses were analyzed by incubation with the S. aureus antigen matrix. IgG binding was detected with IRDye 680-conjugated goat anti-mouse IgG (Li-Cor) and quantified by infrared imaging. This experiment demonstrated that S. aureus WU1 colonization led to increases in serum IgG directed against the sortase-anchored surface proteins ClfA, ClfB, IsdA, and IsdB and the giant extracellular matrix-binding protein (Ebh), a cell size and peptidoglycan synthesis determinant of S. aureus (45) (Table 2).
TABLE 2.
Serum IgG responses in C57BL/6J mice colonized with S. aureus WU1 or its Δspa varianta
| Protein type | Antigen | WU1 (colonized) |
WU1 Δspa |
||||
|---|---|---|---|---|---|---|---|
| Colonized |
Cleared |
||||||
| Fold changeb | Pc (vs naive) | Fold change | P (vs WU1, colonized) | Fold change | P (vs WU1 Δspa, colonized) | ||
| Cell wall anchored surface | SpAKKAA | 1.3 ± 0.08 | NS | 1.1 ± 0.06 | NS | 1.1 ± 0.49 | NS |
| ClfA | 5.3 ± 2.77 | <0.0001 | 4.3 ± 0.83 | NS | 3.5 ± 1.69 | NS | |
| ClfB | 4.8 ± 0.72 | 0.001 | 3.9 ± 1.28 | NS | 17.4 ± 4.70 | <0.0001 | |
| Ebh | 3.7 ± 0.50 | 0.0454 | 2.8 ± 0.62 | NS | 3.9 ± 1.56 | NS | |
| FnbpA | 1.9 ± 0.89 | NS | 1.3 ± 0.79 | NS | 2.6 ± 0.96 | NS | |
| FnbpB | 2.6 ± 1.33 | NS | 2.3 ± 0.85 | NS | 4.3 ± 0.96 | NS | |
| IsdA | 4.5 ± 0.84 | 0.0036 | 2.1 ± 0.22 | NS | 13.0 ± 0.44 | <0.0001 | |
| IsdB | 5.2 ± 1.43 | 0.0002 | 2.7 ± 0.83 | NS | 2.8 ± 1.18 | NS | |
| SdrC | 1.1 ± 0.14 | NS | 1.5 ± 0.45 | NS | 1.7 ± 0.69 | NS | |
| SdrD | 1.5 ± 1.08 | NS | 1.0 ± 0.25 | NS | 1.2 ± 0.35 | NS | |
| SdrE | 1.8 ± 0.52 | NS | 2.9 ± 0.65 | NS | 1.4 ± 0.60 | NS | |
| SasA | 3.0 ± 1.33 | NS | 1.1 ± 0.44 | NS | 3.3 ± 1.14 | NS | |
| SasB | 5.1 ± 2.22 | NS | 1.0 ± 0.34 | NS | 5.7 ± 4.42 | NS | |
| SasD | 2.7 ± 1.47 | NS | 0.7 ± 0.23 | NS | 1.3 ± 0.59 | NS | |
| SasF | 1.2 ± 0.61 | NS | 0.9 ± 0.63 | NS | 1.2 ± 0.32 | NS | |
| SasG | 2.1 ± 0.24 | NS | 1.2 ± 0.47 | NS | 10.3 ± 1.19 | <0.0001 | |
| SasI | 1.4 ± 0.75 | NS | 1.2 ± 0.08 | NS | 1.4 ± 0.53 | NS | |
| SasK | 2.5 ± 0.26 | NS | 1.3 ± 0.30 | NS | 1.7 ± 1.09 | NS | |
| Secreted | Coa | 2.7 ± 0.29 | NS | 1.2 ± 0.45 | NS | 1.5 ± 0.45 | NS |
| vWbp | 2.0 ± 0.97 | NS | 1.4 ± 0.59 | NS | 1.7 ± 0.89 | NS | |
| Hla | 1.8 ± 0.65 | NS | 1.2 ± 0.46 | NS | 1.2 ± 0.34 | NS | |
| SCIN | 4.3 ± 1.23 | 0.0071 | 2.8 ± 1.80 | NS | 1.4 ± 0.49 | NS | |
| Eap | 1.3 ± 0.20 | NS | 0.8 ± 0.97 | NS | 1.2 ± 0.31 | NS | |
| Efb | 2.9 ± 1.68 | NS | 2.6 ± 1.63 | NS | 1.6 ± 0.52 | NS | |
| EsxA | 2.6 ± 1.73 | NS | 1.6 ± 1.00 | NS | 2.6 ± 0.35 | NS | |
| EsxB | 2.8 ± 0.28 | NS | 1.6 ± 0.19 | NS | 1.9 ± 0.21 | NS | |
Cohorts of C57BL/6J mice were inoculated intranasally with 108 CFU of the indicated S. aureus strains. Fifteen days following inoculation, the animals were bled, and serum samples were analyzed for antibody responses to staphylococcal antigens.
Fold changes were calculated by dividing the average signal intensities derived from S. aureus-inoculated mice by the average signal intensities from mice that remained naive. The data are presented as means ± standard deviations.
P values were calculated using two-way ANOVA with Tukey multiple-comparison tests. NS, not significant.
S. aureus WU1 requires staphylococcal protein A for persistent colonization.
Similar to that of S. aureus Newman, the spa gene of S. aureus WU1 encodes the YSIRK/GXXS signal peptide with a signal peptide cleavage site, five IgBDs, a cell wall repeat region (Xr), and a C-terminal LPXTG motif sorting signal. Compared to spa from S. aureus Newman, the coding sequence for the five IgBDs of S. aureus WU1 spa carries a single nucleotide substitution at codon 157 (AAA instead of AAT), replacing asparagine (N) at position 4 in the IgBD-A repeat with lysine (K). Immunoblotting experiments revealed that S. aureus strains Newman and WU1 produced similar amounts of SpA (Fig. 3A). Using allelic recombination, we generated the Δspa mutant of S. aureus WU1. As measured by immunoblotting, SpA production was abolished in the Δspa mutant, and this defect was restored by plasmid-borne expression of wild-type spa (pSpA) (Fig. 3A). Immunoblotting with antibodies against sortase A (SrtA) was used as a loading control (Fig. 3A). When inoculated into the right nostrils of mice and analyzed for colonization by oropharyngeal swabbing on day 7, the Δspa mutant initially colonized C57BL/6 animals in a manner similar to that of wild-type strain WU1 (Fig. 3B). However, at later time points, particularly on days 35 and 42, the Δspa mutant colonized fewer animals than wild-type strain WU1 (Fig. 3B). During bacterial growth, S. aureus releases SpA linked to peptidoglycan fragments into the surrounding milieu (46). In a mouse model of intravenous S. aureus challenge, released SpA activated B cell proliferation and enhanced secretion of VH3 idiotype IgM and IgG molecules (33). However, expanded VH3 idiotype IgG does not recognize staphylococcal antigens (33). The molecular basis for this B cell superantigen activity is based on SpA-mediated cross-linking of VH3 idiotype B cell receptors, which triggers B cell proliferation in a CD4 T helper cell and RIPK2 kinase-dependent manner (33, 47). Animals infected with Δspa mutant staphylococci lack VH3 idiotypic immunoglobulin expansion and exhibit an increased abundance of pathogen-specific IgG, thereby triggering immune responses that are protective against subsequent S. aureus infection (48). We wondered whether colonization with the Δspa mutant of WU1 was associated with altered serum IgG responses. Sera from animals that had been colonized for 15 days were analyzed for IgG binding to components of the S. aureus antigen matrix (Table 2). This experiment revealed increases in antibodies against ClfB, IsdA, and SasG in animals that were subsequently decolonized but not in animals that remained colonized with the Δspa mutant (Table 2). Taken together, these data suggest that nasopharyngeal colonization of C57BL/6 mice with Δspa mutant staphylococci is associated with increased IgG responses against key colonization determinants, which appears to promote removal of Δspa mutant S. aureus from the nasopharynx.
FIG 3.
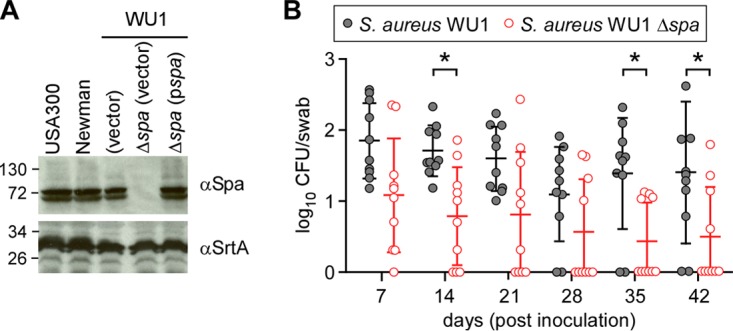
S. aureus WU1 expression of SpA is required for persistent colonization of C57BL/6 mice. (A) Immunoblots of S. aureus lysates derived from strains USA300 LAC, Newman, WU1, and the Δspa variant of WU1 with and without a plasmid for spa expression (pspa) were probed with SpA-specific (αSpA) and sortase A-specific (αSrtA) antibodies. Sizes are shown on the left in kilodaltons. (B) Cohorts of C57BL/6 mice (n = 10) were inoculated intranasally with 1 × 108 CFU of S. aureus WU1 or its Δspa variant, and the oropharynxes of the animals were swabbed at weekly intervals to enumerate the bacterial load. Each dot indicates the number of CFU per mouse. The median and standard deviation for each group of animals on a given day are indicated by the horizontal lines and error bars. The bacterial colonization data sets were analyzed with two-way ANOVA and Sidak multiple-comparison tests; statistically significant differences (*, P < 0.05) between the two groups of animals are indicated.
Protein A-neutralizing antibodies affect persistent colonization with S. aureus.
Immunization of mice with wild-type protein A does not elicit IgG serum antibodies that bind and neutralize the capacity of the five IgBDs of SpA to bind either the Fcγ domain of IgG molecules or the variant heavy chain of VH3 idiotype immunoglobulin (44). SpAKKAA is a variant with 20 amino acid substitutions throughout the five IgBDs of SpA that abolish Fcγ binding and also diminish association with VH3 idiotype immunoglobulin (44). Nevertheless, SpAKKAA retains the overall α-helical content and antigen structure of protein A. As a result, immunization of mice with adjuvanted SpAKKAA elicits high-titer protein A-neutralizing IgG (44). These antibodies block the antiopsonic and B cell superantigen activities of protein A during S. aureus infection, broadly enhancing IgG responses against staphylococcal antigens and promoting the development of protective immunity (44). To test whether protein A-neutralizing antibodies affect S. aureus colonization, C57BL/6 mice were immunized with adjuvanted SpAKKAA or with adjuvant alone. Compared to mock-immunized animals, SpAKKAA-treated animals elicited high-titer protein A-neutralizing antibodies (Table 3). When inoculated with S. aureus WU1, both mock- and SpAKKAA-immunized animals were initially colonized in similar manners, as oropharyngeal swabs revealed average colonizing loads that were not significantly different on days 7 and 14 following inoculation (Fig. 4). However, beginning on day 21, SpAKKAA-immunized mice were more frequently decolonized than mock-immunized animals (Fig. 4). Examination for serum IgG responses and comparison to naive mice showed that S. aureus WU1 colonization in mock-treated animals led to antibody responses against ClfB, IsdA, IsdB, SasD, and SasF (Table 3). In animals that maintained S. aureus WU1 colonization, SpAKKAA immunization led to antibody responses against ClfA, Coa, vWbp, and Hla (Table 3). SpAKKAA-vaccinated C57BL/J mice that were subsequently decolonized exhibited elevated serum IgG against ClfA, ClfB, fibronectin binding proteins A (FnBPA) and B (FnBPB), IsdB, Coa, and SasG (Table 3). Together, these data indicate that SpAKKAA vaccination elicited enhanced serum IgG responses in mice that had been colonized with S. aureus. Further, SpAKKAA vaccine induced antibodies against many different staphylococcal antigens, including known colonization factors (ClfB, IsdA, and SasG). Thus, SpAKKAA vaccine-induced IgG responses against colonizing staphylococci appear to promote decolonization of the nasopharynx.
TABLE 3.
Impact of SpAKKAA immunization on serum IgG responses in S. aureus WU1-colonized C57BL/6 micea
| Protein type | Antigen | SpAKKAA immunized |
PBS mock immunized (colonized) |
||||
|---|---|---|---|---|---|---|---|
| Colonized |
Cleared |
||||||
| Fold changeb |
Pc (vs PBS mock immunized) |
Fold change | P (vs SpAKKAA immunized, colonized) | Fold change | P (vs naive) | ||
| Cell wall anchored surface | SpAKKAA | 121.3 ± 64.98 | <0.0001 | 126.3 ± 13.35 | <0.0001 | 0.9 ± 0.16 | NS |
| ClfA | 3.8 ± 0.49 | <0.0001 | 5.7 ± 2.28 | 0.0069 | 1.3 ± 0.65 | NS | |
| ClfB | 1.1 ± 0.28 | NS | 14.8 ± 1.12 | <0.0001 | 4.3 ± 1.49 | <0.0001 | |
| Ebh | 1.0 ± 0.15 | NS | 1.3 ± 0.57 | NS | 1.3 ± 0.43 | NS | |
| FnbpA | 1.1 ± 0.34 | NS | 6.4 ± 1.86 | <0.0001 | 1.1 ± 0.29 | NS | |
| FnbpB | 1.5 ± 0.33 | NS | 10.6 ± 1.0 | <0.0001 | 1.2 ± 0.72 | NS | |
| IsdA | 1.8 ± 0.46 | NS | 2.8 ± 0.59 | NS | 2.0 ± 0.43 | NS | |
| IsdB | 1.7 ± 0.37 | NS | 5.8 ± 2.75 | <0.0001 | 2.1 ± 0.96 | NS | |
| SdrC | 1.4 ± 0.67 | NS | 1.5 ± 0.61 | NS | 1.2 ± 0.45 | NS | |
| SdrD | 1.1 ± 0.39 | NS | 1.5 ± 0.36 | NS | 1.2 ± 0.23 | NS | |
| SdrE | 1.2 ± 0.36 | NS | 1.8 ± 0.94 | NS | 1.2 ± 0.22 | NS | |
| SasA | 1.8 ± 0.36 | NS | 1.6 ± 0.28 | NS | 0.8 ± 0.80 | NS | |
| SasB | 1.9 ± 0.90 | NS | 1.1 ± 0.42 | NS | 1.0 ± 0.24 | NS | |
| SasD | 1.3 ± 0.46 | NS | 1.0 ± 0.44 | NS | 2.4 ± 0.53 | 0.0023 | |
| SasF | 2.4 ± 0.34 | NS | 1.7 ± 0.55 | NS | 2.6 ± 1.59 | 0.004 | |
| SasG | 0.9 ± 0.15 | NS | 5.5 ± 1.04 | <0.0001 | 1.1 ± 0.32 | NS | |
| SasI | 2.1 ± 0.46 | NS | 1.8 ± 0.02 | NS | 1.3 ± 0.22 | NS | |
| SasK | 2.3 ± 0.62 | NS | 2.7 ± 0.38 | NS | 1.1 ± 0.02 | NS | |
| Secreted | Coa | 3.0 ± 1.31 | 0.0049 | 5.8 ± 0.87 | <0.0001 | 1.2 ± 0.43 | NS |
| vWbp | 5.7 ± 1.34 | <0.0001 | 6.6 ± 2.82 | NS | 1.4 ± 0.65 | NS | |
| Hla | 2.9 ± 0.08 | 0.0070 | 3.6 ± 0.36 | NS | 1.1 ± 0.58 | NS | |
| SCIN | 2.1 ± 0.77 | NS | 1.4 ± 0.21 | NS | 1.0 ± 0.37 | NS | |
| Eap | 1.7 ± 0.38 | NS | 1.1 ± 0.22 | NS | 0.9 ± 0.23 | NS | |
| Efb | 1.5 ± 0.47 | NS | 1.49 ± 0.25 | NS | 0.98 ± 0.27 | NS | |
| EsxA | 2.4 ± 0.65 | NS | 3.22 ± 1.81 | NS | 0.82 ± 0.26 | NS | |
| EsxB | 2.5 ± 0.35 | NS | 3.75 ± 1.08 | NS | 1.46 ± 0.25 | NS | |
Cohorts of C57BL/6J mice were immunized with 50 μg of recombinant SpAKKAA emulsified with CFA or mock immunized with PBS in CFA and boosted on day 11 with 50 μg of recombinant SpAKKAA emulsified with IFA or mock boosted with PBS in IFA. On day 24, the mice were inoculated intranasally with 108 CFU of the indicated S. aureus strains and swabbed in the throat weekly to enumerate the bacterial load. Fifteen days following inoculation, the animals were bled, and serum samples were analyzed for antibody responses to staphylococcal antigens.
Fold changes were calculated by dividing the average signal intensities derived from SpAKKAA-immunized mice by the average signal intensities of PBS mock-immunized mice. The data are presented as means ± standard deviations.
P values were calculated using two-way ANOVA with Tukey multiple-comparison tests. NS, not significant.
FIG 4.
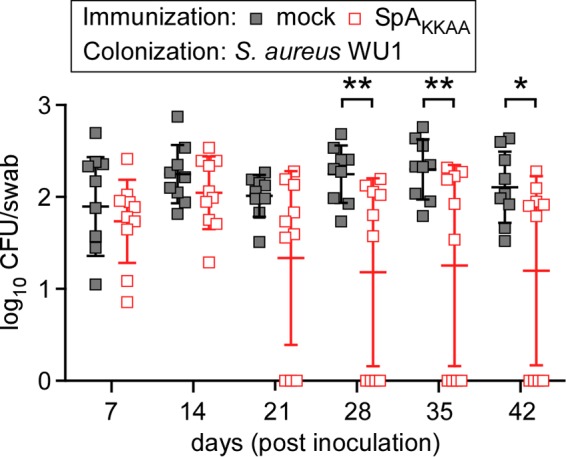
Immunization of C57BL/6 mice with SpAKKAA promotes decolonization of S. aureus WU1. C57BL/6 mice were immunized with 50 μg of purified recombinant SpAKKAA emulsified with CFA or mock immunized with PBS in CFA, boosted after 11 days with 50 μg of recombinant SpAKKAA emulsified with IFA or mock boosted with PBS in IFA, and inoculated with S. aureus 24 days following the initial immunization. On day 0 of the colonization experiment, cohorts of C57BL/6 mice (n = 10) were inoculated intranasally with 1 × 108 CFU of S. aureus WU1. The oropharynxes of the animals were swabbed at weekly intervals to enumerate the bacterial load. Each square indicates the number of CFU for one mouse. The median and standard deviation for each group of animals on a given day are indicated by the horizontal lines and error bars. Bacterial colonization data sets were analyzed with two-way ANOVA and Sidak multiple-comparison tests; statistically significant differences (*, P < 0.05; **, P < 0.01) between the two groups of animals are indicated.
S. aureus WU1 colonization of BALB/c mice.
To test whether S. aureus WU1 colonization was restricted to C57BL/6 mice, we inoculated cohorts (n = 20) of naive BALB/c mice with 1 × 108 CFU S. aureus WU1 into the right nostril and measured nasopharyngeal colonization with swab cultures. Similar to C57BL/6 mice, S. aureus WU1 persistently colonized BALB/c mice (Fig. 5). Immunization of BALB/c mice with SpAKKAA did not affect the initial colonization with S. aureus WU1. However, compared to mock-immunized animals, vaccination with SpAKKAA promoted decolonization of BALB/c mice (Fig. 5).
FIG 5.
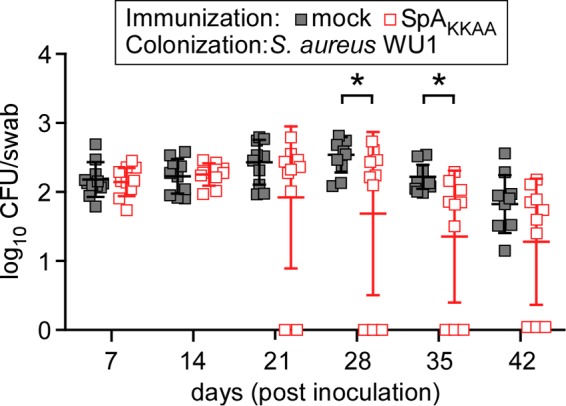
Immunization of BALB/c mice with SpAKKAA promotes decolonization of S. aureus WU1. BALB/c mice were immunized with 50 μg of purified recombinant SpAKKAA emulsified with CFA or mock immunized with PBS in CFA, boosted after 11 days with 50 μg of recombinant SpAKKAA emulsified with IFA or mock boosted with PBS in IFA, and inoculated with S. aureus 24 days following the initial immunization. On day 0 of the colonization experiment, cohorts of BALB/c mice (n = 20) were inoculated intranasally with 1 × 108 CFU of S. aureus WU1. The oropharynxes of the animals were swabbed at weekly intervals to enumerate the bacterial load. Each square indicates the number of CFU for one mouse. The median and standard deviation for each group of animals on a given day are indicated by the horizontal lines and error bars. Bacterial colonization data sets were analyzed with two-way ANOVA and Sidak multiple-comparison tests; statistically significant differences (*, P < 0.05) between groups of animals are indicated.
SpAKKAA vaccine affects mouse colonization with S. aureus JSNZ.
We wondered whether protein A-neutralizing antibodies also affect mouse colonization with S. aureus JSNZ. Unlike those of strains Newman and WU1, the spa gene product of S. aureus JSNZ comprises only four IgBDs (37). Previous work demonstrated that SpA variants with four IgBDs are associated with diminished B cell superantigen activity compared to the five IgBDs generally associated with S. aureus colonization of the human nasopharynx (33). When inoculated into the right nostrils of anesthetized mice, S. aureus JSNZ effectively colonized the nasopharynxes of BALB/c mice over 42 days (Fig. 6). SpAKKAA vaccination did not affect initial colonization with S. aureus JSNZ. However, compared to mock-immunized mice, BALB/c mice with serum neutralizing protein A antibodies more frequently decolonized S. aureus JSNZ starting on day 21 (Fig. 6). Together, these data suggest that S. aureus JSNZ also requires protein A-mediated B cell superantigen activity for persistent colonization of mice.
FIG 6.
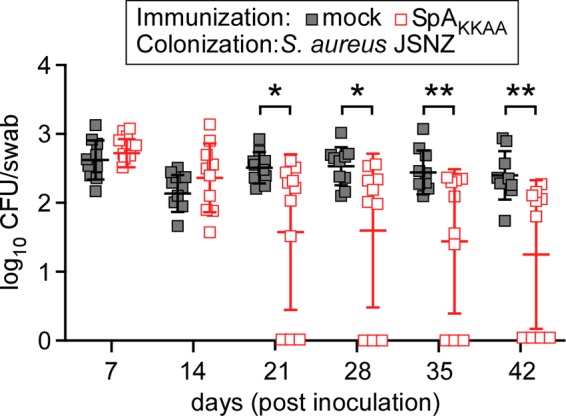
Immunization of BALB/c mice with SpAKKAA promotes S. aureus JSNZ clearance from the nasopharynx. BALB/c mice were immunized with 50 μg of purified recombinant SpAKKAA emulsified with CFA or mock immunized with PBS in CFA, boosted after 11 days with 50 μg of recombinant SpAKKAA emulsified with IFA or mock boosted with PBS in IFA, and inoculated with S. aureus 24 days following the initial immunization. On day 0 of the colonization experiment, cohorts of BALB/c mice (n = 10) were inoculated intranasally with 1 × 108 CFU of S. aureus JSNZ. The oropharynxes of the animals were swabbed at weekly intervals to enumerate the bacterial load. Each square indicates the number of CFU for one mouse. The median and standard deviation for each group of animals on a given day are indicated by the horizontal lines and error bars. The bacterial colonization data sets were analyzed with two-way ANOVA with Sidak multiple-comparison tests; statistically significant differences (*, P < 0.05; **, P < 0.01) between the two groups of animals are indicated.
DISCUSSION
In the United States, S. aureus causes skin and soft tissue infections (SSTI) that result in 14.2 million outpatient visits per year and in more than 850,000 hospital admissions (49–51). S. aureus SSTI are associated with complications such as bacteremia, the need for surgical intervention, and death (52, 53). S. aureus also causes more than 380,000 hospital-acquired infections per year (54). At increased risk of S. aureus infection are patients with surgical procedures, trauma, implantation of foreign bodies, endotracheal intubation, indwelling catheters, or hemodialysis, as well as immunosuppressive or cancer therapies (55). Even with antibiotic and surgical therapy, recurrent disease is a hallmark of S. aureus infection and manifests either as relapse with the index strain or as reinfection with another isolate (56). Relapses occur about four times more frequently than reinfections (57). S. aureus colonization predisposes individuals to both community- and hospital-acquired infections (6, 14, 58).
S. aureus vaccine efficacy trials enrolled hospital patients at high risk of infection, including patients with hemodialysis and patients undergoing open heart surgery (59, 60). Both trials failed to achieve their study endpoints: to diminish the incidence of S. aureus infection (59, 60). Further, immunization with one of the two vaccine candidates, a capsular polysaccharide conjugate, did not affect human colonization with S. aureus (61, 62). In contrast, licensed capsular polysaccharide vaccines that are effective at preventing bacterial meningitis also provide protection from nasopharyngeal colonization with Neisseria meningitidis, which is a key risk factor for invasive disease (63, 64). Moreover, N. meningitidis colonization eventually leads to the development of natural immunity via bactericidal, serotype-specific capsular polysaccharide antibodies that prevent further colonization with strains of the same serotype (65). In contrast, S. aureus colonization of humans is not associated with the development of bactericidal or opsonizing IgG antibodies that can prevent further colonization or invasive disease (2).
Our work has been focused on characterizing the S. aureus determinant responsible for suppressing adaptive immune responses. We hypothesized that the underlying mechanism may support persistent colonization of humans and also prevent the development of immunity to invasive disease (66, 67). SpA, a molecule that impacts B cell development and antibody production, is expressed by all clinical S. aureus isolates (29, 32). SpA is secreted and anchored to the bacterial wall so that its N-terminal IgBDs (58- to 62-residue E, D, B, C, and A domains with a triple-helical fold) are displayed on the bacterial surface while the C-terminal end is linked to peptidoglycan (68–70). During growth, S. aureus murein hydrolases cut peptidoglycan and release SpA into the extracellular milieu to modify host B cell responses (33, 46). The IgBDs of SpA molecules on the staphylococcal surface bind to the Fcγ portion of human immunoglobulin (IgG1, IgG2, IgG4, and IgA) and block the effector functions of antibodies, which otherwise would engage Fcγ and complement receptors on phagocytes (71, 72). Released SpA binds and cross-links the variant heavy chain of VH3 idiotype B cell receptors (IgM) (73, 74). SpA cross-linking promotes proliferation of VH3 idiotype B cells and secretion of IgG and IgA molecules that are affinity matured for SpA binding yet lack specificity for S. aureus antigens (33, 75). SpA B cell superantigen activity diverts adaptive immune responses during invasive disease in a mouse bacteremia model with naive animals (48). In contrast, mice infected with an isogenic Δspa mutant exhibit increased adaptive immune responses to staphylococcal disease and protection against subsequent infection (48). During S. aureus infection in humans, mice, or guinea pigs, the unique functional attributes of SpA also prevent the development of antibodies that can bind and neutralize its IgBDs (44, 48, 76). Here, we asked whether spa expression is required for S. aureus colonization, using a mouse model with an ST88 isolate that has evolved to persistently colonize laboratory mice and cause invasive disease.
Earlier work used S. aureus Newman, specifically, a streptomycin-resistant variant, to analyze nasal colonization in streptomycin-treated mice (19). In this model, S. aureus Newman adheres to mouse nasal epithelia and replicates for 14 days but is cleared by 21 days (21). The importance of surface proteins for attachment to nasal epithelia and for colonization was demonstrated with a sortase mutant (ΔsrtA) that cannot anchor surface proteins with LPXTG-sorting signals (SpA, ClfA, ClfB, FnBPA, FnBPB, SdrC, SdrD, SdrE, IsdA, IsdB, SasG, etc.) to the bacterial envelope (77). The ΔsrtA variant of S. aureus Newman exhibited a colonization defect 14 days after inoculation (78). A phenotype of diminished bacterial load in the nasal cavities of mice was also observed with the S. aureus Newman ΔclfB mutant (78). Further, ClfB-specific IgG antibodies, whether elicited by active immunization or passively transferred to naive mice, reduced staphylococcal loads in mouse nasal tissue but did not diminish colonization (78). ClfB binds to the α-chain of human and mouse fibrinogen and to the Y(GS)nY quasirepeats of loricrin and cytokeratin K10 (21, 79, 80). Whereas wild-type FVB mice were effectively colonized by S. aureus Newman over 10 days, loricrin knockout mice were partially decolonized over the same period (21). The contributions of IsdA and SasG to S. aureus colonization have not yet been studied in mice. In the cotton rat model, IsdA certainly contributed to S. aureus nasal colonization, and IsdA-specific antibodies provided partial protection against colonization (81). Thus, S. aureus colonization of the nasopharynxes of mice and humans likely requires the functions of multiple surface proteins, and IgG antibodies against the molecules likely impact the bacterial load and/or persistence of staphylococci in these tissues.
The isolation of S. aureus strains that persistently colonize the nasopharynx and gastrointestinal tract in laboratory mice offers unique research opportunities to systematically identify staphylococcal determinants of nasal attachment, colonization, and persistence in the host (36, 37). Further, animal models of persistent colonization can be used for vaccine studies by analyzing long-term immune responses during colonization and invasive disease, the contributions of specific immune-evasive factors, and interference of vaccines with either colonization or disease (36). Here, we contribute to this burgeoning field by isolating the mouse-adapted strain S. aureus WU1. Similar to isolates reported previously, S. aureus WU1 belongs to the CC88 clade. We noted the presence of vwb alleles that appeared to promote enhanced agglutination of mouse plasma by S. aureus WU1 and JSNZ. Earlier work highlighted the contributions of host-specific vwb alleles to S. aureus adaptation to ruminant and equine hosts (82). Thus, host-specific adaptation of vwb appears to be a universal feature of S. aureus evolution, supporting the unique pathogenesis of its abscess lesions (83). We show further that spa is not required for the initial colonization of mice with S. aureus WU1, indicating that protein A is not a colonization factor sensu stricto. However, starting 14 days after the initial inoculation, mice inoculated with spa mutants are more frequently decolonized than control animals carrying wild-type staphylococci. Decolonization coincides with the development of serum IgG against S. aureus colonization factors. SpA-neutralizing antibodies, generated via SpAKKAA immunization, achieve similar decolonization in mice. Such decolonization was also associated with the development of IgG specific for S. aureus colonization factors (ClfB, IsdA, and SasG). Together, these data suggest that the B cell superantigen activity of protein A may prevent adaptive immune responses against bacterial secreted products, thereby enabling persistent colonization with S. aureus. We note that some, but not all, animals were decolonized in a protein A-dependent manner over the 42-day time course of our experiments. It seems plausible that prolonged colonization (for 60 or more days) with spa mutant staphylococci or SpA-neutralizing antibodies may generate further increases in serum IgG responses that achieve increased decolonization. Alternatively, some hosts may require episodes of invasive disease to formulate adaptive immune responses that result in decolonization of the pathogen. Nevertheless, to our knowledge, these data provide the first experimental evidence in support of the hypothesis that SpA B cell superantigen activity contributes to the persistent S. aureus colonization of mice.
Unlike subunit vaccines that are comprised of individual or combinations of protective antigens, SpAKKAA is a vaccine that activates antibody responses against all antigens of S. aureus that are recognizable by the immune system. This effect, however, can be achieved only when individuals with SpA-neutralizing antibodies encounter the pathogen (44, 84). Previous work demonstrated the vaccine effects of SpA-neutralizing antibodies in mice and guinea pigs with S. aureus bloodstream infections (44, 76). Here, we demonstrate that the vaccine effect of SpAKKAA immunization can also be implemented in individuals that are colonized with S. aureus. The superantigen activity and immune-evasive attributes of SpA are confined to B cells with VH3 idiotype IgM receptors, which, owing to the germ line repertoire of immunoglobulin variable heavy chain genes, are highly abundant in humans (>50%) but much less abundant in guinea pigs (20 to 25% of blood B cells) and mice (10% of blood B cells) (48, 73). We therefore speculate that the vaccine efficacy of nontoxigenic protein A vaccines for S. aureus colonization may be greater in humans than in mice.
MATERIALS AND METHODS
Media and bacterial growth conditions.
S. aureus strain WU1 was isolated in the animal facility at Washington University, St. Louis, MO. S. aureus strains were propagated in tryptic soy broth (TSB) or on tryptic soy agar (TSA) at 37°C. For experiments investigating mouse nasopharyngeal colonization, throat swab samples were grown on Baird-Parker agar at 37°C as indicated. For experiments investigating S. aureus GI tract colonization, stool samples were grown on mannitol salt agar at 37°C as indicated. Escherichia coli strains DH5α and BL21(DE3) were grown in Luria broth (LB) or agar at 37°C. Ampicillin (100 μg/ml for E. coli) and chloramphenicol (10 μg/ml for S. aureus) were used for plasmid selection.
S. aureus genotyping.
S. aureus isolate WU1 was obtained from the nasopharynxes and preputial gland abscess lesions of mice in our animal facility. Mouse S. aureus strain JSNZ was provided by Siouxsie Wiles (36). Staphylococcal genomic DNA was isolated with the Wizard genomic DNA purification kit (Promega). Spa genotyping and MLST were performed as previously described (85). Briefly, for spa typing, the genomic DNA of S. aureus strain WU1 was PCR amplified with primers 1095F (5′-AGACGATCCTTCGGTGAGC-3′) and 1517R (5′-GCTTTTGCAATGTCATTTACTG-3′) (86). The PCR product was purified with the Nucleospin gel and PCR cleanup kit, sequenced with primers 1095F and 1517R, and analyzed with Ridom software (http://spaserver.ridom.de/spa-t186.shtml). For MLST, the genomic DNA of S. aureus strain WU1 was PCR amplified with primers arc-up (5′-TTGATTCACCAGCGCGTATTGTC-3′), arc-dn (5′-AGGTATCTGCTTCAATCAGCG-3′), aro-up (5′-ATCGGAAATCCTATTTCACATTC-3′), aro-dn (5′-GGTGTTGTATTAATAACGATATC-3′), glp-up (5′-CTAGGAACTGCAATCTTAATCC-3′), glp-dn (5′-TGGTAAAATCGCATGTCCAATTC-3′), gmk-up (5′-ATCGTTTTATCGGGACCATC-3′), gmk-dn (5′-TCATTAACTACAACGTAATCGTA-3′), pta-up (5′-GTTAAAATCGTATTACCTGAAGG-3′), pta-dn (5′-GACCCTTTTGTTGAAAAGCTTAA-3′), tpi-up (5′-TCGTTCATTCTGAACGTCGTGA-3′), tpi-dn (5′-TTTGCACCTTCTAACAATTGTAC-3′), yqi-up (5′-CAGCATACAGGACACCTATTGGC-3′), and yqi-dn (5′-CGTTGAGGAATCGATACTGGAAC-3′) (http://saureus.mlst.net/misc/info.asp). The PCR product was purified with the Nucleospin gel and PCR cleanup kit, PCR amplified, and sequenced and analyzed with on-line software (http://saureus.mlst.net/sql/multiplelocus.asp). Whole-genome sequence files for S. aureus strain JSNZ were provided by Silva Holtfreter. TruSeq DNA-seq library preparation Illumina MiSeq sequencing was performed with the genomic DNA of S. aureus WU1 by the Environmental Sample Preparation and Sequencing Facility at Argonne National Laboratory. Sequences were analyzed using Geneious software.
S. aureus mutants.
Allelic recombination with the plasmid pKOR1 was used to delete the spa gene of S. aureus WU1 (87). To construct the Δspa mutant, two 1-kb DNA fragments upstream and downstream of the spa gene were amplified from the chromosome of S. aureus WU1 with primers ext1F (5′-GGGGACCACTTTGTACAAGAAAGCTGGGTCATTTAAGAAGATTGTTTCAGATTTATG-3′), ext1R (5′-ATTTGTAAAGTCATCATAATATAACGAATTATGTATTGCAATACTAAAATC-3′), ext2F (5′-CGTCGCGAACTATAATAAAAACAAACAATACACAACGATAGATATC-3′), and ext2R (5′-GGGGACAAGTTTGTACAAAAAAGCAGGCAACGAACGCCTAAAGAAATTGTCTTTGC-3′). The two flanking regions were fused in a subsequent PCR, and the final PCR product was cloned into pKOR1 using the BP Clonase II kit (Invitrogen). The resulting plasmids were consecutively transferred into E. coli DH5α, S. aureus strain RN4220, and finally S. aureus strain WU1 and temperature shifted to 40°C, blocking replication of the plasmids and promoting their insertion into the chromosome (87). Growth at 30°C was used to promote allelic replacement. Mutations in the spa genes were verified by DNA sequencing of the PCR amplification products.
Agglutination assay.
Agglutination assays were performed as previously described (88). Briefly, overnight cultures of S. aureus strains were diluted 1:100 in fresh TSB and grown at 37°C for 6 h. Bacteria from 1 ml of culture (normalized to an optical density at 600 nm [OD600] of 4.0) were incubated with Syto 9 (1:500; Invitrogen) for 15 min, washed twice with 1 ml PBS, and suspended in 1 ml PBS. The bacteria were mixed 1:1 with citrate-treated human plasma or mouse plasma on glass microscope slides and incubated for 30 min. Samples were viewed and images were captured on an IX81 live-cell total internal reflection fluorescence microscope using a 20× objective (Olympus). At least 10 images were acquired for each sample. The areas of agglutination complexes in each image were measured and quantified using ImageJ software.
Immunoblotting.
Overnight cultures of S. aureus strains were diluted 1:100 in fresh TSB (with chloramphenicol in the presence of plasmids) and grown at 37°C to an OD600 of 0.5 to 1.0. Cells from 1 ml of culture were centrifuged, suspended in PBS, and incubated with 20 μg/ml lysostaphin (AMBI) at 37°C for 1 h. Proteins in the whole-cell lysate were precipitated with 10% trichloroacetic acid and 10 μg deoxycholic acid, washed with ice-cold acetone, air dried, suspended in 100 μl 0.5 M Tris·HCl (pH 6.8) and 100 μl SDS-PAGE sample buffer (100 mM Tris-HCl [pH 6.8], 4% SDS, 0.2% bromophenol blue, 200 mM dithiothreitol), and boiled for 10 min. The proteins were separated on SDS-12% PAGE and electrotransferred to a polyvinylidene difluoride (PVDF) membrane. The PVDF membranes were blocked with 5% milk in Tris-buffered saline with Tween 20 (TBST) (20 mM Tris-HCl [pH 7.6], 137 mM NaCl, 0.1% Tween 20). Mouse anti-ClfA 2A12.12 monoclonal antibody (1:2,000 dilution) and horseradish peroxidase (HRP)-conjugated anti-mouse IgG (Cell Signaling; 1:10,000 dilution) were used to detect ClfA. Rabbit anti-Coa polyclonal antibody (1:1,000 dilution) and HRP-conjugated anti-rabbit IgG (1:10,000 dilution) were used to detect Coa. Two different rabbit anti-vWbp polyclonal antibodies (1:1,000 dilution), which recognize full-length vWbp from S. aureus Newman or the C-terminal domain of vWbp, respectively, and HRP-conjugated anti-rabbit IgG (1:10,000 dilution) were used to detect vWbp. HRP-conjugated human IgM in TBST (1:10,000 dilution) was used to detect SpA. Rabbit anti-SrtA polyclonal antibodies (1:10,000 dilution) and HRP-conjugated anti-rabbit IgG (1:10,000 dilution) were used to detect SrtA. Antibody-stained membranes were washed with TBST, incubated with SuperSignal West Pico chemiluminescent substrate (Thermo Scientific), and developed on Amersham Hyperfilm ECL high-performance chemiluminescence films (GE Healthcare).
Purification of recombinant proteins.
E. coli BL21(DE3) harboring pET15b+ plasmids for the expression of His-tagged SpAKKAA, as well as 25 staphylococcal antigens (ClfA, ClfB, FnBPA, FnBPB, IsdA, IsdB, SasA, SasB, SasD, SasF, SasG, SasI, SasK, SdrC, SdrD, SdrE, EsxA, EsxB, SCIN, Eap, Efb, Hla, Coa, vWbp, and Ebh), was grown overnight, diluted 1:100 in fresh medium, and grown at 37°C to an OD600 of ∼0.5. The cultures were induced with 1 mM isopropyl-β-d-thiogalactopyranoside and grown for an additional 3 h. The cells were pelleted, resuspended in column buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl), and disrupted with a French pressure cell at 14,000 lb/in2. The lysates were cleared of membrane and insoluble components by ultracentrifugation at 40,000 × g. The cleared lysates were subjected to Ni-nitrilotriacetic acid (Ni-NTA) affinity chromatography, and proteins were eluted in column buffer containing successively higher concentrations of imidazole (100 to 500 mM). The eluates were dialyzed with PBS, and the protein purity was verified by Coomassie-stained SDS-PAGE. Protein concentrations were determined by bicinchoninic acid assay (Thermo Scientific).
Mouse nasopharyngeal colonization.
Overnight cultures of S. aureus strain WU1 and its Δspa mutant were diluted 1:100 in fresh TSB and grown for 2 h at 37°C. The cells were centrifuged, washed, and suspended in PBS. Seven-week-old female BALB/c, C57BL/6J, or B6.129S2-Ighmtm1Cgn/J mice (Jackson Laboratory) were anesthetized by intraperitoneal injection with 100 mg/ml ketamine and 20 mg/ml xylazine per kilogram of body weight, and 1 × 108 CFU of S. aureus (in a 10-μl volume) was pipetted into the right nostril of each mouse. On days 7, 14, 21, 28, 35, and 42 following inoculation, the oropharynxes of the mice were swabbed, and swab samples were spread on BPA and incubated for bacterial enumeration. On day 15 following inoculation, the mice were bled via periorbital vein puncture to obtain sera for antibody response analyses using the staphylococcal antigen matrix. On day 42 following inoculation, stool samples were collected and homogenized in PBS. The homogenates were plated on MSA and incubated for bacterial enumeration. All mouse experiments were performed in accordance with the institutional guidelines following experimental protocol review and approval by the Institutional Biosafety Committee (IBC) and the Institutional Animal Care and Use Committee (IACUC) at the University of Chicago. Animal experiments were repeated at least once to ensure reproducibility of the data.
Active immunization.
Four-week-old mice were immunized by subcutaneous injection with 50 μg of SpAKKAA emulsified in complete Freund's adjuvant (CFA) (Difco) and boosted with 50 μg of the same antigen emulsified in incomplete Freund's adjuvant (IFA) 11 days following the initial immunization. On day 21, the immunized mice were bled via periorbital vein puncture to obtain sera for enzyme-linked immunosorbent assay (ELISA). On day 24, the mice were inoculated intranasally with 1 × 108 CFU of S. aureus strain WU1 or JSNZ and monitored for nasopharyngeal colonization.
Staphylococcal antigen matrix.
Nitrocellulose membranes were blotted with 2 μg affinity-purified staphylococcal antigens. The membranes were blocked with 5% degranulated milk and incubated with diluted mouse sera (1:10,000 dilution) and IRDye 680-conjugated goat anti-mouse IgG (Li-Cor). Signal intensities were quantified using the Odyssey infrared imaging system (Li-Cor).
Statistical analysis.
Two-way analysis of variance (ANOVA) with Sidak multiple-comparison tests (GraphPad Software) was performed to analyze the statistical significance of nasopharyngeal colonization, ELISA, and antigen matrix data.
ACKNOWLEDGMENTS
We thank Michael Diamond and Tiffany Lucas (both from Washington University) for generously providing S. aureus WU1 and members of our laboratory for discussions.
This work was supported by grants AI038897 and AI052474 from the National Institute of Allergy and Infectious Diseases (NIAID), Infectious Diseases Branch.
Y.S., C.E., D.M., and O.S. declare competing interests as inventors of patents and intellectual property filings for vaccines that promote S. aureus decolonization.
REFERENCES
- 1.Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VGJ. 2015. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Belkum A, Melles DC, Nouwen J, van Leeuwen WB, van Wamel W, Vos MC, Wertheim HF, Verbrugh HA. 2009. Co-evolutionary aspects of human colonisation and infection by Staphylococcus aureus. Infect Genet Evol 9:32–47. doi: 10.1016/j.meegid.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Hanssen AM, Kindlund B, Stenklev NC, Furberg AS, Fismen S, Olsen RS, Johannessen M, Solid JUE. 2017. Localization of Staphylococcus aureus in tissue from the nasal vestibule in healthy carriers. BMC Microbiol 17:89. doi: 10.1186/s12866-017-0997-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mertz D, Frei R, Periat N, Zimmerli M, Battegay M, Flückiger U, Widmer AF. 2009. Exclusive Staphylococcus aureus throat carriage: at-risk populations. Arch Intern Med 169:172–178. doi: 10.1001/archinternmed.2008.536. [DOI] [PubMed] [Google Scholar]
- 5.Boyce JM, Havill NL, Maria B. 2005. Frequency and possible infection control implications of gastrointestinal colonization with methicillin-resistant Staphylococcus aureus. J Clin Microbiol 43:5992–5995. doi: 10.1128/JCM.43.12.5992-5995.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Eiff C, Becker K, Machka K, Stammer H, Peters G. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med 344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 7.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 8.Buehlmann M, Frei R, Fenner L, Dangel M, Fluckiger U, Widmer AF. 2008. Highly effective regimen for decolonization of methicillin-resistant Staphylococcus aureus carriers. Infect Control Hosp Epidemiol 29:510–516. doi: 10.1086/588201. [DOI] [PubMed] [Google Scholar]
- 9.Gilpin DF, Small S, Bakkshi S, Kearney MP, Cardwell C, Tunney MM. 2010. Efficacy of a standard methicillin-resistant Staphylococcus aureus decolonisation protocol in routine clinical practice. J Hosp Infect 75:93–98. doi: 10.1016/j.jhin.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 10.Acton DS, Plat-Sinnige MJ, van Wamel W, de Groot N, van Belkum A. 2009. Intestinal carriage of Staphylococcus aureus: how does its frequency compare with that of nasal carriage and what is its clinical impact? Eur J Clin Microbiol Infect Dis 28:115–127. doi: 10.1007/s10096-008-0602-7. [DOI] [PubMed] [Google Scholar]
- 11.Verkaik NJ, Lebon A, de Vogel CP, Hooijkaas H, Verbrugh HA, Jaddoe VW, Hofman A, Moll HA, van Belkum A, van Wamel WJ. 2010. Induction of antibodies by Staphylococcus aureus nasal colonization in young children. Clin Microbiol Infect 16:1312–1317. doi: 10.1111/j.1469-0691.2009.03073.x. [DOI] [PubMed] [Google Scholar]
- 12.Swierstra J, Debets S, de Vogel C, Lemmens-den Toom N, Verkaik N, Ramdani-Bouguessa N, Jonkman MF, van Dijl JM, Fahal A, van Belkum A, van Wamel W. 2015. IgG4 subclass-specific responses to Staphylococcus aureus antigens shed new light on host-pathogen interaction. Infect Immun 83:492–501. doi: 10.1128/IAI.02286-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holtfreter S, Jursa-Kulesza J, Masiuk H, Verkaik NJ, de Vogel C, Kolata J, Nowosiad M, Steil L, van Wamel W, van Belkum A, Völker U, Giedrys-Kalemba S, Bröker BM. 2011. Antibody responses in furunculosis patients vaccinated with autologous formalin-killed Staphylococcus aureus. Eur J Clin Microbiol Infect Dis 30:707–717. doi: 10.1007/s10096-010-1136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kluytmans J, van Belkum A, Verburgh H. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 10:505–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinstein HJ. 1959. The relation between nasal-staphylococcal-carrier state and the incidence of postoperative complications. N Engl J Med 260:1303–1308. doi: 10.1056/NEJM195906252602601. [DOI] [PubMed] [Google Scholar]
- 16.Missiakas D, Schneewind O. 2016. Staphylococcus aureus vaccines: deviating from the carol. J Exp Med 213:1645–1653. doi: 10.1084/jem.20160569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corrigan RM, Miajlovic H, Foster TJ. 2009. Surface proteins that promote adherence of Staphylococcus aureus to human desquamated nasal epithelial cells. BMC Microbiol 9:22. doi: 10.1186/1471-2180-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weidenmaier C, Kokai-Kun JF, Kristian SA, Chanturiya T, Kalbacher H, Gross M, Nicholson G, Neumeister B, Mond JJ, Peschel A. 2004. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat Med 10:243–245. doi: 10.1038/nm991. [DOI] [PubMed] [Google Scholar]
- 19.Kiser KB, Cantey-Kiser JM, Lee JC. 1999. Development and characterization of a Staphylococcus aureus nasal colonization model in mice. Infect Immun 67:5001–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weidenmaier C, Goerke C, Wolz C. 2012. Staphylococcus aureus determinants for nasal colonization. Trends Microbiol 20:243–250. doi: 10.1016/j.tim.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Mulcahy ME, Geoghegan JA, Monk IR, O'Keeffe KM, Walsh EJ, Foster TJ, McLoughlin RM. 2012. Nasal colonisation by Staphylococcus aureus depends upon clumping factor B binding to the squamous epithelial cell envelope protein loricrin. PLoS Pathog 8:e1003092. doi: 10.1371/journal.ppat.1003092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke SR, Wiltshire MD, Foster SJ. 2004. IsdA of Staphylococcus aureus is a broad spectrum, iron-regulated adhesin. Mol Microbiol 51:1509–1519. doi: 10.1111/j.1365-2958.2003.03938.x. [DOI] [PubMed] [Google Scholar]
- 23.Mazmanian SK, Skaar EP, Gaspar AH, Humayun M, Gornicki P, Jelenska J, Joachmiak A, Missiakas DM, Schneewind O. 2003. Passage of heme-iron across the envelope of Staphylococcus aureus. Science 299:906–909. doi: 10.1126/science.1081147. [DOI] [PubMed] [Google Scholar]
- 24.Clarke SR, Foster SJ. 2008. IsdA protects Staphylococcus aureus against the bactericidal protease activity of apolactoferrin. Infect Immun 76:1518–1526. doi: 10.1128/IAI.01530-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conrady DG, Brescia CC, Horii K, Weiss AA, Hassett DJ, Herr AB. 2008. A zinc-dependent adhesion molecule is responsible for intercellular adhesion in staphylococcal biofilms. Proc Natl Acad Sci U S A 105:19456–19461. doi: 10.1073/pnas.0807717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roche FM, Meehan M, Foster TJ. 2003. The Staphylococcus aureus surface protein SasG and its homologues promote bacterial adherence to human desquamated nasal epithelial cells. Microbiology 149:2759–2767. doi: 10.1099/mic.0.26412-0. [DOI] [PubMed] [Google Scholar]
- 27.Baur S, Rautenberg M, Faulstich M, Grau T, Severin Y, Unger C, Hoffmann WH, Rudel T, Autenrieth IB, Weidenmaier C. 2014. A nasal epithelial receptor for Staphylococcus aureus WTA governs adhesion to epithelial cells and modulates nasal colonization. PLoS Pathog 10:e1004089. doi: 10.1371/journal.ppat.1004089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winstel V, Kühner P, Salomon F, Larsen J, Skov R, Hoffmann W, Peschel A, Weidenmaier C. 2015. Wall teichoic acid glycosylation governs Staphylococcus aureus nasal colonization. mBio 6:e00632–e00615. doi: 10.1128/mBio.00632-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forsgren A. 1970. Significance of protein A production by staphylococci. Infect Immun 2:672–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burian M, Wolz C, Goerke C. 2010. Regulatory adaptation of Staphylococcus aureus during nasal colonization of humans. PLoS One 5:e10040. doi: 10.1371/journal.pone.0010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenkins A, Diep BA, Mai TT, Vo NH, Warrener P, Suzich J, Stover CK, Sellman BR. 2015. Differential expression and roles of Staphylococcus aureus virulence determinants during colonization and disease. mBio 6:e02272–e02214. doi: 10.1128/mBio.02272-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Votintseva AA, Fung R, Miller RR, Knox K, Godwin H, Wyllie DH, Bowden R, Crook DW, Walker AS. 2014. Prevalence of Staphylococcus aureus protein A (spa) mutants in the community and hospitals in Oxfordshire. BMC Microbiol 14:63. doi: 10.1186/1471-2180-14-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HK, Falugi F, Missiakas D, Schneewind O. 2016. Peptidoglycan-linked protein A promotes T-cell dependent antibody expansion during Staphylococcus aureus infection. Proc Natl Acad Sci U S A 113:5718–5723. doi: 10.1073/pnas.1524267113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cole AL, Muthukrishnan G, Chong C, Beavis A, Eade CR, Wood MP, Deichen MG, Cole AM. 2016. Host innate inflammatory factors and staphylococcal protein A influence the duration of human Staphylococcus aureus nasal carriage. Mucosal Immunol 9:1537–1548. doi: 10.1038/mi.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong X, Qin J, Li T, Dai Y, Wang Y, Liu Q, He L, Lu H, Gao Q, Lin Y, Li M. 2016. Staphylococcal protein A promotes colonization and immune evasion of the epidemic healthcare-associated MRSA ST239. Front Microbiol 7:951. doi: 10.3389/fmicb.2016.00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holtfreter S, Radcliff FJ, Grumann D, Read H, Johnson S, Monecke S, Ritchie S, Clow F, Goerke C, Bröker BM, Fraser JD, Wiles S. 2013. Characterization of a mouse-adapted Staphylococcus aureus strain. PLoS One 8:e71142. doi: 10.1371/journal.pone.0071142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulz D, Grumann D, Trübe P, Pritchett-Corning K, Johnson S, Reppschläger K, Gumz J, Sundaramoorthy N, Michalik S, Berg S, van den Brandt J, Fister R, Monecke S, Uy B, Schmidt F, Bröker BM, Wiles S, Holtfreter S. 2017. Laboratory mice are frequently colonized with Staphylococcus aureus and mount a systemic immune response—note of caution for in vivo infection experiments. Front Cell Infect Microbiol 7:152. doi: 10.3389/fcimb.2017.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Wamel WJ, Rooijakkers SH, Ruyken M, van Kessel KP, van Strijp JA. 2006. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on β-hemolysin-converting bacteriophages. J Bacteriol 188:1310–1315. doi: 10.1128/JB.188.4.1310-1315.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jongerius I, Köhl J, Pandey MK, Ruyken M, van Kessel KPM, van Strijp JAG, Rooijakkers SHM. 2007. Staphylococcal complement evasion by various convertase-blocking molecules. J Exp Med 204:2461–2471. doi: 10.1084/jem.20070818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng AG, McAdow M, Kim HK, Bae T, Missiakas DM, Schneewind O. 2010. Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLoS Pathog 6:e1001036. doi: 10.1371/journal.ppat.1001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McAdow M, Kim HK, DeDenta AC, Hendrickx APA, Schneewind O, Missiakas DM. 2011. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog 7:e1002307. doi: 10.1371/journal.ppat.1002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDevitt D, Francois P, Vaudaux P, Foster TJ. 1994. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol 11:237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 43.Baba T, Bae T, Schneewind O, Takeuchi F, Hiramatsu K. 2008. Genome sequence of Staphylococcus aureus strain Newman and comparative analysis of staphylococcal genomes. J Bacteriol 190:300–310. doi: 10.1128/JB.01000-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HK, Cheng AG, Kim H-Y, Missiakas DM, Schneewind O. 2010. Non-toxigenic protein A vaccine for methicillin-resistant Staphylococcus aureus infections. J Exp Med 207:1863–1870. doi: 10.1084/jem.20092514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng AG, Missiakas DM, Schneewind O. 2014. The giant protein Ebh is a cross wall determinant of Staphylococcus aureus cell size and complement resistance. J Bacteriol 196:971–981. doi: 10.1128/JB.01366-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Becker S, Frankel MB, Schneewind O, Missiakas DM. 2014. Release of protein A from the cell wall envelope of Staphylococcus aureus. Proc Natl Acad Sci U S A 111:1574–1579. doi: 10.1073/pnas.1317181111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodyear CS, Silverman GJ. 2004. Staphylococcal toxin induced preferential and prolonged in vivo deletion of innate-like B lymphocytes. Proc Natl Acad Sci U S A 101:11392–11397. doi: 10.1073/pnas.0404382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Falugi F, Kim HK, Missiakas DM, Schneewind O. 2013. The role of protein A in the evasion of host adaptive immune responses by Staphylococcus aureus. mBio 4:e00575–e00513. doi: 10.1128/mBio.00575-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hersh AL, Chambers HF, Maselli JH, Gonzales R. 2008. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 168:1585–1591. doi: 10.1001/archinte.168.14.1585. [DOI] [PubMed] [Google Scholar]
- 50.Pallin DJ, Egan DJ, Pelletier AJ, Espinola JA, Hooper DC, Camargo CA Jr. 2008. Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med 51:291–298. doi: 10.1016/j.annemergmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Edelsberg J, Taneja C, Zervos M, Haque N, Moore C, Reyes K, Spalding J, Jiang J, Oster G. 2009. Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis 15:1516–1518. doi: 10.3201/eid1509.081228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lipsky BA, Kollef MH, Miller LG, Sun X, Johannes RS, Tabak YP. 2010. Predicting bacteremia among patients hospitalized for skin and skin-structure infections: derivation and validation of a risk score. Infect Control Hosp Epidemiol 31:828–837. doi: 10.1086/654007. [DOI] [PubMed] [Google Scholar]
- 53.Carratalà J, Rosón B, Fernández-Sabé N, Shaw E, del Rio O, Rivera A, Gudiol F. 2003. Factors associated with complications and mortality in adult patients hospitalized for infectious cellulitis. Eur J Clin Microbiol Infect Dis 22:151–157. [DOI] [PubMed] [Google Scholar]
- 54.Lucero CA, Hageman J, Zell ER, Bulens S, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Schaffner W, Fridkin SK. 2009. Evaluating the potential public health impact of a Staphylococcus aureus vaccine through use of population-based surveillance for invasive methicillin-resistant S. aureus disease in the United States. Vaccine 27:5061–5068. doi: 10.1016/j.vaccine.2009.06.055. [DOI] [PubMed] [Google Scholar]
- 55.Spellberg B, Daum RS. 2012. Development of a vaccine against Staphylococcus aureus. Semin Immunopathol 34:335–348. doi: 10.1007/s00281-011-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Creech CB, Al-Zubeidi DN, Fritz SA. 2015. Prevention of recurrent staphylococcal skin infections. Infect Dis Clin North Am 29:429–464. doi: 10.1016/j.idc.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fowler VG Jr, Kong LK, Corey GR, Gottlieb GS, McClelland RS, Sexton DJ, Gesty-Palmer D, Harrell LJ. 1999. Recurrent Staphylococcus aureus bacteremia: pulsed-field gel electrophoresis findings in 29 patients. J Infect Dis 179:1157–1161. doi: 10.1086/314712. [DOI] [PubMed] [Google Scholar]
- 58.Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JA, van Keulen PH, Vandenbroucke-Grauls CM, Meester MH, Verbrugh HA. 2004. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364:703–705. doi: 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]
- 59.Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, Yeoh H, Law D, Robbins JB, Schneerson R, Muenz L, Fuller S, Johnson J, Fireman B, Alcorn H, Naso R. 2002. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med 346:491–496. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 60.Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, Corey GR, Carmeli Y, Betts R, Hartzel JS, Chan IS, McNeely TB, Kartsonis NA, Guris D, Onorato MT, Smugar SS, DiNubile MJ, Sobanjo-ter Meulen A. 2013. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 309:1368–1378. doi: 10.1001/jama.2013.3010. [DOI] [PubMed] [Google Scholar]
- 61.Fattom AI, Horwith G, Fuller S, Propst M, Naso R. 2004. Development of StaphVAX, a polysaccharide conjugate vaccine against S. aureus infection: from the lab bench to phase III clinical trials. Vaccine 22:880–887. doi: 10.1016/j.vaccine.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 62.Creech CB, Johnson BG, Alsentzer AR, Hohenboken M, Edwards KM, Talbot TR. 2009. Vaccination as infection control: a pilot study to determine the impact of Staphylococcus aureus vaccination on nasal carriage. Vaccine 28:256–260. doi: 10.1016/j.vaccine.2009.09.088. [DOI] [PubMed] [Google Scholar]
- 63.Gotschlich EC, Goldschneider I, Artenstein MS. 1969. Human immunity to the meningococcus. V. The effect of immunization with meningococcal group C polysaccharide on the carrier state. J Exp Med 129:1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goldschneider I, Gotschlich EC, Artenstein MS. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med 129:1307–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goldschneider I, Gotschlich EC, Artenstein MS. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med 129:1327–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim HK, Thammavongsa V, Schneewind O, Missiakas D. 2012. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr Opin Microbiol 15:92–99. doi: 10.1016/j.mib.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thammavongsa V, Kim HK, Missiakas DM, Schneewind O. 2015. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol 13:529–543. doi: 10.1038/nrmicro3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.DeDent AC, McAdow M, Schneewind O. 2007. Distribution of protein A on the surface of Staphylococcus aureus. J Bacteriol 189:4473–4484. doi: 10.1128/JB.00227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sjöquist J, Movitz J, Johansson I-B, Hjelm H. 1972. Localization of protein A in the bacteria. Eur J Biochem 30:190–194. doi: 10.1111/j.1432-1033.1972.tb02086.x. [DOI] [PubMed] [Google Scholar]
- 70.Schneewind O, Fowler A, Faull KF. 1995. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science 268:103–106. doi: 10.1126/science.7701329. [DOI] [PubMed] [Google Scholar]
- 71.Forsgren A. 1968. Protein A from Staphylococcus aureus. VI. Reaction with subunits from guinea pig γ1- and γ2-globulin. J Immunol 100:927–930. [PubMed] [Google Scholar]
- 72.Forsgren A, Quie PG. 1974. Effects of staphylococcal protein A on heat labile opsonins. J Immunol 112:1177–1180. [PubMed] [Google Scholar]
- 73.Goodyear CS, Silverman GJ. 2003. Death by a B cell superantigen: in vivo VH-targeted apoptotic supraclonal B cell deletion by a staphylococcal toxin. J Exp Med 197:1125–1139. doi: 10.1084/jem.20020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silverman GJ, Goodyear CS. 2006. Confounding B-cell defences: lessons from a staphylococcal superantigen. Nat Rev Immunol 6:465–475. doi: 10.1038/nri1853. [DOI] [PubMed] [Google Scholar]
- 75.Pauli NT, Kim HK, Falugi F, Huang M, Dulac J, Dunand CH, Zheng NY, Kaur K, Andrews S, Huang Y, Dedent A, Frank K, Charnot-Katsikas A, Schneewind O, Wilson PC. 2014. Staphylococcus aureus infection induces protein A-mediated immune evasion in humans. J Exp Med 211:2331–2339. doi: 10.1084/jem.20141404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim HK, Falugi F, Thomer L, Missiakas DM, Schneewind O. 2015. Protein A suppresses immune responses during Staphylococcus aureus bloodstream infection in guinea pigs. mBio 6:e02369–e02314. doi: 10.1128/mBio.02369-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mazmanian SK, Liu G, Jensen ER, Lenoy E, Schneewind O. 2000. Staphylococcus aureus mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc Natl Acad Sci U S A 97:5510–5515. doi: 10.1073/pnas.080520697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schaffer AC, Solinga RM, Cocchiaro J, Portoles M, Kiser KB, Risley A, Randall SM, Valtulina V, Speziale P, Walsh E, Foster T, Lee JC. 2006. Immunization with Staphylococcus aureus clumping factor B, a major determinant in nasal carriage, reduces nasal colonization in a murine model. Infect Immun 74:2145–2153. doi: 10.1128/IAI.74.4.2145-2153.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walsh EJ, O'Brien LM, Liang X, Hook M, Foster TJ. 2004. Clumping factor B, a fibrinogen-binding MSCRAMM (microbial surface components recognizing adhesive matrix molecules) adhesin of Staphylococcus aureus, also binds to the tail region of type I cytokeratin 10. J Biol Chem 279:50691–50699. doi: 10.1074/jbc.M408713200. [DOI] [PubMed] [Google Scholar]
- 80.Ní Eidhin D, Perkins S, Francois P, Vaudaux P, Höök M, Foster TJ. 1998. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol Microbiol 30:245–257. doi: 10.1046/j.1365-2958.1998.01050.x. [DOI] [PubMed] [Google Scholar]
- 81.Clarke SR, Brummell KJ, Horsburgh MJ, McDowell PW, Mohamad SA, Stapleton MR, Acevedo J, Read RC, Day NP, Peacock SJ, Mond JJ, Kokai-Kun JF, Foster SJ. 2006. Identification of in vivo-expressed antigens of Staphylococcus aureus and their use in vaccinations for protection against nasal carriage. J Infect Dis 193:1098–1108. doi: 10.1086/501471. [DOI] [PubMed] [Google Scholar]
- 82.Viana D, Blanco J, Tormo-Más MA, Selva L, Guinane CM, Baselga R, Corpa JM, Lasa I, Novick RP, Fitzgerald JR, Penadés JR. 2010. Adaptation of Staphylococcus aureus to ruminant and equine hosts involves SaPI-carried variants of von Willebrand factor-binding protein. Mol Microbiol 77:1583–1594. doi: 10.1111/j.1365-2958.2010.07312.x. [DOI] [PubMed] [Google Scholar]
- 83.Cheng AG, DeDent AC, Schneewind O, Missiakas DM. 2011. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol 19:225–232. doi: 10.1016/j.tim.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim HK, Emolo C, DeDent AC, Falugi F, Missiakas DM, Schneewind O. 2012. Protein A-specific monoclonal antibodies and the prevention of Staphylococcus aureus disease in mice. Infect Immun 80:3460–3470. doi: 10.1128/IAI.00230-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chambers HF, DeLeo FR. 2009. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harmsen D, Claus H, Witte W, Rothgänger J, Claus H, Turnwald D, Vogel U. 2003. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol 41:5442–5448. doi: 10.1128/JCM.41.12.5442-5448.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bae T, Schneewind O. 2006. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55:58–63. doi: 10.1016/j.plasmid.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 88.Thomer L, Becker S, Emolo C, Quach A, Kim HK, Rauch S, Anderson M, Leblanc JF, Schneewind O, Faull KF, Missiakas D. 2014. N-Acetylglucosaminylation of serine-aspartate repeat proteins promotes Staphylococcus aureus bloodstream infection. J Biol Chem 289:3478–3486. doi: 10.1074/jbc.M113.532655. [DOI] [PMC free article] [PubMed] [Google Scholar]


