Abstract
Background
Immunotherapies have demonstrated efficacy across a diverse set of tumors supporting further evaluation in glioblastoma. The objective of this study was to evaluate the safety/tolerability and describe immune-mediated effects of nivolumab ± ipilimumab in patients with recurrent glioblastoma. Exploratory efficacy outcomes are also reported.
Methods
Patients were randomized to receive nivolumab 3 mg/kg every 2 weeks (Q2W; NIVO3) or nivolumab 1 mg/kg + ipilimumab 3 mg/kg every 3 weeks (Q3W) for 4 doses, then nivolumab 3 mg/kg Q2W (NIVO1+IPI3). An alternative regimen of nivolumab 3 mg/kg + ipilimumab 1 mg/kg Q3W for 4 doses, then nivolumab 3 mg/kg Q2W (NIVO3+IPI1) was investigated in a nonrandomized arm.
Results
Forty patients were enrolled (NIVO3, n = 10; NIVO1+IPI3, n = 10; NIVO3+IPI1, n = 20). The most common treatment-related adverse events (AEs) were fatigue (NIVO3, 30%; NIVO1+IPI3, 80%; NIVO3+IPI1, 55%) and diarrhea (10%, 70%, 30%, respectively). AEs leading to discontinuation occurred in 10% (NIVO3), 30% (NIVO1+IPI3), and 20% (NIVO3+IPI1) of patients. Three patients achieved a partial response (NIVO3, n = 1; NIVO3+IPI1, n = 2) and 8 had stable disease for ≥12 weeks (NIVO3, n = 2; NIVO1+IPI3, n = 2; NIVO3+IPI1, n = 4 [Response Assessment in Neuro-Oncology criteria]). Most patients (68%) had tumor-cell programmed death ligand-1 expression ≥1%. Immune-mediated effects mimicking radiographic progression occurred in 2 patients.
Conclusions
Nivolumab monotherapy was better tolerated than nivolumab + ipilimumab; the tolerability of the combination was influenced by ipilimumab dose. These safety and exploratory findings merit further investigation of immunotherapies in glioblastoma.
Keywords: CheckMate 143, ipilimumab, nivolumab, PD-L1, recurrent glioblastoma
Importance of the study
Glioblastoma tumors generate an immunosuppressive environment through several mechanisms (eg, programmed death 1/cytotoxic T-lymphocyte antigen 4/indoleamine 2,3-dioxygenase 1/lymphocyte-activation gene 3 pathways) that help them avoid the immune system. Immune checkpoint inhibitors have provided clinical benefit in multiple malignancies, including disease control in melanoma brain metastases. Therefore, checkpoint inhibitors could have a role in difficult-to-treat patients with glioblastoma. In this first article reporting a prospective trial of immune checkpoint inhibitors in recurrent glioblastoma, nivolumab ± ipilimumab regimens were tolerable, with no unexpected treatment-related neurological events reported. Exploratory efficacy results indicated that ~20% of patients achieved stable disease ≥12 weeks, and 5 (12.5%) survived >25 months. Pathologically assessed treatment-related changes in some patients were indistinguishable from radiological progression, highlighting the difficulties of assessing progression by MRI. Signs of antitumor immune responses were observed in some patients, indicating that immunotherapies may overcome the immunosuppressive CNS environment in selected patients. Larger studies investigating the optimal role of immunotherapy in glioblastoma are ongoing.
Glioblastoma (GBM), the most common malignant primary brain tumor in adults, is a highly invasive and aggressive form of cancer.1,2 Patients with GBM have a poor prognosis, with 5-year survival rates of 5%–10%.1,3 Initial treatment for patients with newly diagnosed disease usually involves surgical resection followed by radiotherapy with concomitant and adjuvant temozolomide.4 However, nearly all tumors recur, and available salvage therapies such as temozolomide, bevacizumab, lomustine, and tumor-treating fields have limited efficacy, providing a median overall survival (OS) of 5.4–10.6 months.4–10 Due to the severity of this disease and the lack of effective treatment options, further investigation of novel therapies is needed.
Many tumors attempt to evade the immune response by exploiting immune regulatory checkpoints, including the immunosuppressive cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death 1 (PD-1) receptor pathways.2 Ipilimumab is a fully human immunoglobulin G subclass 1 monoclonal antibody that inhibits CTLA-4 with demonstrated efficacy in metastatic melanoma.11 Nivolumab is a fully human immunoglobulin G subclass 4 monoclonal antibody inhibitor of PD-1, approved globally for the treatment of multiple malignancies12,13; nivolumab has demonstrated clinical benefit (improved survival and/or rate of response) compared with former standard-of-care treatments in all approved indications.14–23
Although the CNS historically has been considered an immuno-privileged site, increasing evidence suggests that the CNS interacts with the peripheral immune system and is immunocompetent.2,24 Expression of the PD-1 ligand (PD-L1) has been observed in primary GBM tumors, and expression levels were found to correlate with glioma grade.25,26 Furthermore, treatment with immune checkpoint inhibitors demonstrated improved survival in murine models of glioma27 and was able to provide disease control (stable disease or better) in some patients with melanoma who had brain metastases,28,29 suggesting that immunotherapy could be a potential treatment option for patients with CNS tumors.
Here we report the results of the first prospective clinical trial of immune checkpoint inhibitors in recurrent GBM. This phase I study evaluated the safety, tolerability, and immunologic effects of nivolumab with or without ipilimumab at different dose levels in patients with recurrent GBM, with the goal of guiding larger randomized studies in this disease. Selected efficacy outcomes were also assessed in an exploratory analysis.
Materials and Methods
This report describes results (data cutoff: January 20, 2017) from the lead-in, exploratory phase I cohorts of CheckMate 143 (NCT02017717). These cohorts evaluated the safety of nivolumab with or without ipilimumab at 2 different dosing schedules; the study was conducted at 9 sites in the United States. All enrolled patients provided written informed consent, and the study protocol was approved by the institutional review board or independent ethics committee of each participating institution.
Patients
Eligible patients were ≥18 years old at the time of screening and had a histologically confirmed diagnosis of World Health Organization (WHO) grade IV malignant glioma30 (GBM or gliosarcoma), irrespective of O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status and tumor PD-L1 expression, and a KPS of ≥70%. Patients had a first recurrence of GBM following first-line treatment with at least radiotherapy and temozolomide, confirmed by diagnostic biopsy or contrast-enhanced MRI ≤21 days prior to study entry per Response Assessment in Neuro-Oncology (RANO) criteria.31 For cases in which MRI was used to document recurrence, an interval of ≥12 weeks following radiotherapy was required unless new enhancement outside of the radiotherapy treatment field was reported or histopathologic confirmation of recurrent tumor was obtained. Patients were included if they had at least 1 measurable lesion with at least 2 perpendicular enhancing diameters measuring ≥10 mm. Patients were excluded if they had more than 1 recurrence of GBM; diagnosis of secondary GBM; evidence of extracranial metastatic or leptomeningeal disease; active, known, or suspected autoimmune disease; or prior treatment with an anti–PD-1 or anti–CTLA-4 therapy. Concomitant medications are described in the Supplementary material.
Study Design
Patients were initially randomized (1:1) to receive either nivolumab 3 mg/kg every 2 weeks (Q2W; NIVO3) or nivolumab 1 mg/kg + ipilimumab 3 mg/kg every 3 weeks (Q3W) for 4 doses followed by nivolumab 3 mg/kg Q2W thereafter (NIVO1+IPI3). A subsequent nonrandomized cohort in which patients received nivolumab 3 mg/kg + ipilimumab 1 mg/kg Q3W for 4 doses followed by nivolumab 3 mg/kg Q2W thereafter (NIVO3+IPI1) was initiated due to results in other tumor types suggesting that NIVO3+IPI1 may be better tolerated than NIVO1+IPI332; this cohort started enrolling patients after accrual to the randomized cohort was completed.
Patients were treated until confirmed disease progression, intolerable toxicity, or withdrawal of consent. Dose reductions were not permitted. Safety assessments were performed continuously during treatment and for ≥100 days after the last dose of study treatment or until a treatment-related adverse event (TRAE) resolved, returned to baseline, or was deemed irreversible. AEs and laboratory values were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.0.33
Tumor assessments were performed using contrast-enhanced MRI every 6 weeks for the first 2 assessments, followed by every 8 weeks thereafter or as clinically indicated according to RANO criteria. Radiological response was assessed by comparing the baseline and on-treatment MRI scans, and progression was determined by using the smallest tumor measurement at baseline or after initiation of study medication. Patients who met radiological criteria for disease progression were permitted to remain on study medication at the discretion of the treating physician if a clinical benefit was indicated and treatment was tolerated, or until progression was confirmed by MRI or surgery. If the follow-up assessment confirmed progression, then progression was recorded as having occurred on the date of initial determination. Survival was assessed throughout treatment and every 3 months during the follow-up phase.
Tumor tissue was collected from all patients at baseline (archival [at initial diagnosis] or fresh [at recurrence]) and from patients who had suspected progression at the time of on-study surgery or biopsy; tissue collected from patients who had suspected progression was retrospectively assessed by central neuropathological review. Hematoxylin and eosin staining and immunohistochemistry analyses were performed using formalin-fixed, paraffin-embedded tumor tissue. For biomarker analyses assessing expression of PD-L1, the Dako IHC 28-8 pharmDx assay (rabbit anti-human antibody clone 28-8) was used. Tumor PD-L1 expression was evaluated by the frequency of tumor cells expressing membrane PD-L1 (ie, patients with PD-L1 expression levels ≥1% were defined as those with ≥1 PD-L1–positive tumor cell within a field of 100 evaluable cells). PD-L1 analyses were performed using archival or fresh tissue, and samples were considered evaluable for PD-L1 expression if there was sufficient tissue to perform the assay.
Outcomes
The primary objective was to evaluate the safety and tolerability of nivolumab with or without ipilimumab in patients with recurrent GBM. Exploratory objectives included evaluation of the objective response rate (confirmed complete response or partial response) per RANO criteria, progression-free survival (PFS), and determination of the frequency of PD-L1 expression in recurrent GBM. Although OS was not included among the prespecified outcome assessments, it was analyzed as an ad hoc exploratory objective.
Statistical Analyses
The primary endpoint of safety and tolerability was analyzed based on AEs and laboratory parameters that were graded per CTCAE v4.0. Exploratory endpoints of PFS and OS were analyzed by Kaplan–Meier estimates, using the product-limit method, and reported with 2-sided 95% CIs. PFS was calculated from the date of treatment initiation to the date of progression or death, whichever occurred first.
Role of the Funding Source
The study was designed by the authors in collaboration with the sponsor (Bristol-Myers Squibb). The authors and the sponsor were responsible for data collection, and the sponsor was responsible for data analysis. The authors and sponsor were involved in data interpretation and the development of this report, and they attest that the study was conducted in accordance with the study protocol.
Results
Patient Characteristics and Disposition
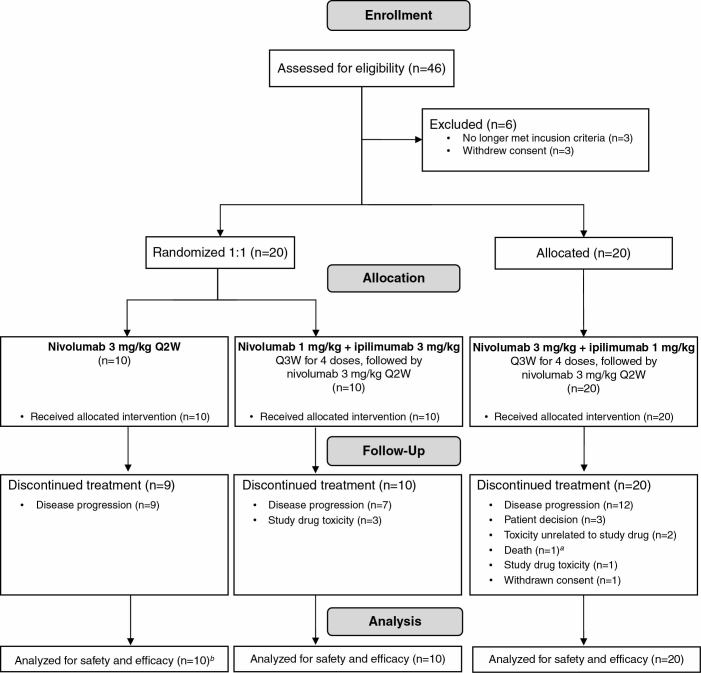
Between February 2014 and September 2014, 40 patients were treated in the study; the first 20 patients enrolled were randomized to receive either NIVO3 (n = 10) or NIVO1+IPI3 (n = 10), and the following 20 patients were assigned to receive NIVO3+IPI1 (Fig. 1). Baseline characteristics were comparable between treatment groups (Table 1). All treated patients had a histopathologic diagnosis of GBM (except 1 patient [NIVO3], who had a diagnosis of gliosarcoma) and at least 1 measurable lesion (except 1 patient [NIVO3], who had only a nonmeasurable lesion [patient not evaluable for response]).
Fig. 1.
Trial profile. aPatient died due to a stroke 39 days after the last dose of study treatment. bOne patient did not have a measurable target lesion at baseline and therefore was not evaluable for response; this patient was evaluable for PFS and OS.
Table 1.
Patient demographics and clinical characteristics
| Characteristic | NIVO3 (n = 10) |
NIVO1+IPI3 (n = 10) |
NIVO3+IPI1 (n = 20) |
|---|---|---|---|
| Age | |||
| Median, y (range) | 58.5 (42–73) | 57 (37–68) | 60 (27–73) |
| <65, n (%) | 6 (60) | 7 (70) | 17 (85) |
| ≥65 to <75, n (%) | 4 (40) | 3 (30) | 3 (15) |
| Sex, n (%) | |||
| Male | 5 (50) | 6 (60) | 14 (70) |
| Female | 5 (50) | 4 (40) | 6 (30) |
| Race, n (%) | |||
| White | 8 (80) | 10 (100) | 18 (90) |
| Black | 2 (20) | 0 | 0 |
| Asian | 0 | 0 | 1 (5) |
| Other/unknown | 0 | 0 | 1 (5) |
| KPS, n (%) | |||
| 90% | 7 (70) | 6 (60) | 11 (55) |
| 80% | 1 (10) | 1 (10) | 5 (25) |
| 70% | 2 (20) | 3 (30) | 4 (20) |
| Histopathologic diagnosis, n (%) | |||
| Glioblastoma | 9 (90) | 10 (100) | 20 (100) |
| Gliosarcoma | 1 (10) | 0 | 0 |
| MGMT promoter methylation status, n (%) | |||
| Methylated | 2 (20) | 2 (20) | 7 (35) |
| Unmethylated | 4 (40) | 6 (60) | 10 (50) |
| Not reported | 4 (40) | 2 (20) | 3 (15) |
| Steroid use, n (%) | |||
| Yes | 2 (20) | 4 (40) | 6 (30) |
| No | 8 (80) | 6 (60) | 14 (70) |
| Median time from initial diagnosis to recurrent diagnosis, mo (range) | 9.7 (3.7–48.9) | 8.4 (5.1–23.0) | 11.35 (4.9–32.9) |
| Patients with ≥1 measurable target lesion, n (%) | 9 (90) | 10 (100) | 20 (100) |
| Sum of reference diameters of target lesions, mm 2 (range) | 903 (120–1664) | 660 (273–1856) | 607 (143–3552) |
| Patients with ≥2 lesions, n (%)a | 4 (40) | 1 (10) | 5 (25) |
| Patients with evaluable PD-L1 expression, n (%)b | 10 (100) | 9 (90) | 18 (90) |
| PD-L1 expression levelsc | |||
| <1% | 3 (30) | 4 (44) | 5 (28) |
| ≥1% | 7 (70) | 5 (56) | 13 (72) |
| ≥10% | 4 (40) | 1 (11) | 5 (28) |
Abbreviations: MGMT, O6-methylguanine-DNA methyltransferase; NIVO3, nivolumab 3 mg/kg; NIVO1+IPI3, nivolumab 1 mg/kg + ipilimumab 3 mg/kg; NIVO3+IPI1, nivolumab 3 mg/kg + ipilimumab 1 mg/kg; ; PD-L1, programmed death ligand 1. aIncludes target and nontarget lesions. bPD-L1 analyses were performed using archival (at initial diagnosis) or fresh (at recurrence) tissue. cPercentages are based on the number of patients with evaluable PD-L1 expression.
As of the data cutoff (January 20, 2017), most patients had discontinued treatment (NIVO3, 90%; NIVO1+IPI3, 100%; NIVO3+IPI1, 100%; Fig. 1). The most common reasons for discontinuation included disease progression (NIVO3, 90%; NIVO1+IPI3, 70%; NIVO3+IPI1, 60%), treatment-related toxicity (NIVO1+IPI3, 30%; NIVO3+IPI1, 5%), patient decision (NIVO3+IPI1, 15%), and toxicity unrelated to study drug (NIVO3+IPI1, 10%). The median durations of follow-up for surviving patients treated with NIVO3 (n = 1) and those treated with NIVO3+IPI1 (n = 3) were 33.7 and 27.2 months (range, 9.2–28.3), respectively.
Treatment Exposure
The median (range) durations of nivolumab treatment in the NIVO3, NIVO1+IPI3, and NIVO3+IPI1 treatment arms were 2.3 (1.0–33.7), 2.2 (0.7–5.9), and 2.45 (0–24.0) months, respectively (Supplementary Table S1). Among patients receiving NIVO1+IPI3 and NIVO3+IPI1, the median (range) durations of ipilimumab treatment were 1.15 (0.7–2.8) and 2.1 (0–3.0) months, respectively (Supplementary Table S1).
Safety
The most common TRAEs in the NIVO3, NIVO1+IPI3, and NIVO3+IPI1 treatment arms were fatigue, diarrhea, headache, increased lipase, and nausea (Table 2). In addition to headache, the only other neurological TRAE occurring in ≥2 patients in any arm was dizziness. One patient receiving NIVO3+IPI1 experienced treatment-related vasogenic cerebral edema. No grade 3/4 TRAEs were reported with NIVO3; however, grade 3/4 events were reported in 9 (90%) and 6 (30%) patients in the NIVO1+IPI3 and NIVO3+IPI1 treatment groups, respectively, with the most common being increased alanine aminotransferase (ALT), increased aspartate aminotransferase (AST), colitis, diarrhea, fatigue, and increased lipase. Treatment-related serious AEs (TRSAEs) were reported in 20% (NIVO3), 70% (NIVO1+IPI3), and 25% (NIVO3+IPI1) of patients (Table 2); increased ALT, increased AST, colitis, diarrhea, hypothyroidism, and pneumonitis were the only TRSAEs that occurred in >1 patient. No grade 5 TRAEs or TRSAEs were reported. AEs leading to discontinuation occurred in 1 patient (10%) receiving NIVO3, 3 patients (30%) receiving NIVO1+IPI3, and 4 patients (20%) receiving NIVO3+IPI1, with increased lipase and muscular weakness leading to treatment discontinuation in more than 1 patient (Supplementary Table S2).
Table 2.
Treatment-related adverse events reported in at least 2 patients (any arm) and treatment-related serious adverse eventsa
| Treatment-Related Adverse Events |
NIVO3
(n = 10) |
NIVO1+IPI3
(n = 10) |
NIVO3+IPI1
(n = 20) |
|||
|---|---|---|---|---|---|---|
| Any Grade | Grade 3/4 | Any Grade | Grade 3/4 | Any Grade | Grade 3/4 | |
| Any TRAE, n (%) | 9 (90) | 0 | 10 (100) | 9 (90) | 20 (100) | 6 (30) |
| TRAEs reported in ≥2 patients in any arm, n (%) | ||||||
| Neurological disorders | ||||||
| Headache | 2 (20) | 0 | 3 (30) | 0 | 4 (20) | 0 |
| Dizziness | 1 (10) | 0 | 0 | 0 | 3 (15) | 1 (5) |
| Gastrointestinal disorders | ||||||
| Diarrhea | 1 (10) | 0 | 7 (70) | 3 (30) | 6 (30) | 1 (5) |
| Nausea | 3 (30) | 0 | 3 (30) | 0 | 3 (15) | 0 |
| Vomiting | 1 (10) | 0 | 4 (40) | 0 | 3 (15) | 0 |
| Colitis | 0 | 0 | 2 (20) | 2 (20) | 1 (5) | 1 (5) |
| General disorders and administration-site conditions | ||||||
| Fatigue | 3 (30) | 0 | 8 (80) | 1 (10) | 11 (55) | 3 (15) |
| Asthenia | 0 | 0 | 0 | 0 | 3 (15) | 0 |
| Gait disturbance | 2 (20) | 0 | 0 | 0 | 1 (5) | 0 |
| Pyrexia | 0 | 0 | 1 (10) | 0 | 2 (10) | 0 |
| Investigations | ||||||
| Lipase increased | 2 (20) | 0 | 5 (50) | 5 (50) | 2 (10) | 0 |
| ALT increased | 0 | 0 | 4 (40) | 2 (20) | 4 (20) | 2 (10) |
| AST increased | 0 | 0 | 5 (50) | 1 (10) | 3 (15) | 2 (10) |
| Amylase increased | 1 (10) | 0 | 3 (30) | 1 (10) | 1 (5) | 0 |
| Platelet count decreased | 0 | 0 | 0 | 0 | 3 (15) | 0 |
| Lymphocyte count decreased | 0 | 0 | 0 | 0 | 2 (10) | 0 |
| Skin and subcutaneous tissue disorders | ||||||
| Rash | 2 (20) | 0 | 1 (10) | 0 | 5 (25) | 0 |
| Pruritus | 2 (20) | 0 | 0 | 0 | 5 (25) | 0 |
| Maculopapular rash | 0 | 0 | 4 (40) | 0 | 0 | 0 |
| Endocrine disorders | ||||||
| Hyperthyroidism | 1 (10) | 0 | 3 (30) | 0 | 1 (5) | 0 |
| Hypothyroidism | 2 (20) | 0 | 1 (10) | 0 | 1 (5) | 0 |
| Blood and lymphatic system disorders | ||||||
| Anemia | 0 | 0 | 0 | 0 | 2 (10) | 0 |
| Eye disorders | ||||||
| Optic nerve disorder | 2 (20) | 0 | 0 | 0 | 0 | 0 |
| Metabolism and nutrition disorders | ||||||
| Appetite decreased | 0 | 0 | 2 (20) | 1 (10) | 1 (5) | 0 |
| Psychiatric disorders | ||||||
| Confusional state | 1 (10) | 0 | 2 (20) | 1 (10) | 2 (10) | 0 |
| Respiratory, thoracic, and mediastinal disorders | ||||||
| Cough | 0 | 0 | 1 (10) | 0 | 2 (10) | 0 |
| Any TRSAE, n (%) | 2 (20) | 0 | 7 (70) | 7 (70) | 5 (25) | 2 (10) |
| Neurological disorders | ||||||
| Headache | 0 | 0 | 0 | 0 | 1 (5) | 0 |
| Gastrointestinal disorders | ||||||
| Colitis | 0 | 0 | 2 (20) | 2 (20) | 1 (5) | 1 (5) |
| Diarrhea | 0 | 0 | 2 (20) | 2 (20) | 1 (5) | 1 (5) |
| Pancreatitis | 0 | 0 | 1 (10) | 1 (10) | 0 | 0 |
| Vomiting | 0 | 0 | 0 | 0 | 1 (5) | 0 |
| Investigations | ||||||
| ALT increased | 0 | 0 | 2 (20) | 2 (20) | 1 (5) | 1 (5) |
| AST increased | 0 | 0 | 1 (10) | 1 (10) | 1 (5) | 1 (5) |
| Lipase increased | 0 | 0 | 1 (10) | 1 (10) | 0 | 0 |
| Bilirubin increased | 0 | 0 | 1 (10) | 0 | 0 | 0 |
| Metabolism and nutrition disorders | ||||||
| Appetite decreased | 0 | 0 | 1 (10) | 1 (10) | 0 | 0 |
| Diabetic ketoacidosis | 0 | 0 | 1 (10) | 1 (10) | 0 | 0 |
| Hyperglycemia | 0 | 0 | 1 (10) | 1 (10) | 0 | 0 |
| Hypocalcemia | 0 | 0 | 1 (10) | 1 (10) | 0 | 0 |
| Hypomagnesemia | 0 | 0 | 1 (10) | 0 | 0 | 0 |
| Endocrine disorders | ||||||
| Hypothyroidism | 1 (10) | 0 | 1 (10) | 0 | 0 | 0 |
| Autoimmune thyroiditis | 0 | 0 | 0 | 0 | 1 (5) | 0 |
| Hyperthyroidism | 0 | 0 | 1 (10) | 0 | 0 | 0 |
| General disorders and administration-site conditions | ||||||
| Chest pain | 1 (10) | 0 | 0 | 0 | 0 | 0 |
| Hepatobiliary disorders | ||||||
| Cholecystitis | 0 | 0 | 1 (10) | 1 (10) | 0 | 0 |
| Infections and infestations | ||||||
| Sepsis | 0 | 0 | 1 (10) | 1 (10) | 0 | 0 |
| Psychiatric disorders | ||||||
| Confusional state | 0 | 0 | 1 (10) | 1 (10) | 0 | 0 |
| Renal and urinary disorders | ||||||
| Acute kidney injury | 0 | 0 | 1 (10) | 1 (10) | 0 | 0 |
| Respiratory, thoracic, and mediastinal disorders | ||||||
| Pneumonitis | 1 (10) | 0 | 0 | 0 | 1 (5) | 0 |
| Skin and subcutaneous tissue disorders | ||||||
| Dermatitis bullous | 0 | 0 | 1 (10) | 0 | 0 | 0 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; NIVO3, nivolumab 3 mg/kg; NIVO1+IPI3, nivolumab 1 mg/kg + ipilimumab 3 mg/kg; NIVO3+IPI1, nivolumab 3 mg/kg + ipilimumab 1 mg/kg; TRAE; treatment-related adverse event; TRSAE, treatment-related serious adverse event. aAdverse events were reported during treatment and for ≥100 days following study drug discontinuation and were evaluated according to the CommonTerminology Criteria for Adverse Events v4.0. Adverse events were sorted based on the Medical Dictionary for Regulatory Activities (MedDRA) groupings.
Nine, 10, and 17 patients died in the NIVO3, NIVO1+IPI3, and NIVO3+IPI1 treatment groups, respectively; of these patients, 2 in each of the NIVO3 and NIVO1+IPI3 groups and 7 in the NIVO3+IPI1 group died ≤100 days after their last dose of study drug. The primary cause of death was disease progression (NIVO3, n = 8 [80%]; NIVO1+IPI3, n = 10 [100%]; NIVO3+IPI1, n = 15 [75%]); 1 patient treated with NIVO3 died due to unknown reasons and 2 patients treated with NIVO3+IPI1 died due to stroke and acute hypoxic respiratory failure (1 each; both deemed unrelated to study drug).
Exploratory Efficacy
Among response-evaluable patients treated with NIVO3 (n = 9), NIVO1+IPI3 (n = 10), and NIVO3+IPI1 (n = 20), 3 patients (NIVO3, n = 1; NIVO3+IPI1, n = 2) achieved a confirmed partial response per investigator assessment according to RANO criteria; the objective response rates in the NIVO3 and NIVO3+IPI1 treatment arms were 11% (95% CI, 0.3–48.2) and 10% (1.2–31.7), respectively (Table 3). Each of the 3 patients with a partial response had a methylated MGMT promoter status and the times from their initial diagnosis to first recurrence (ie, prior to study treatment) were 4.7 (NIVO3), 8.9 (NIVO3+IPI1), and 13.7 months (NIVO3+IPI1). Twelve patients were receiving steroids at baseline (all ≤4 mg/day [dexamethasone equivalents]), of whom 1 (8%; NIVO3+IPI1) achieved a partial response; 2 of 28 patients (7%; NIVO3 and NIVO3+IPI1, n = 1 each) who were not receiving steroids at baseline responded to treatment. Among all patients, stable disease for ≥12 weeks was reported in 2 (22%), 2 (20%), and 4 (20%) patients treated with NIVO3, NIVO1+IPI3, and NIVO3+IPI1, respectively; in these patients, stable disease lasted from 12.2 to 124.3 weeks (NIVO3), 13.0 to 15.2 weeks (NIVO1+IPI3), and 12.2 to 25.2 weeks (NIVO3+IPI1). Disease progression occurred in 4 (44%), 7 (70%), and 9 (45%) patients treated with NIVO3, NIVO1+IPI3, and NIVO3+IPI1, respectively.
Table 3.
Investigator-assessed best overall response and objective response rate
| Response |
NIVO3
(n = 9) |
NIVO1+IPI3
(n = 10) |
NIVO3+IPI1
(n = 20) |
|---|---|---|---|
| Best overall response, n (%)a | |||
| Complete response | 0 | 0 | 0 |
| Partial response | 1 (11) | 0 | 2 (10) |
| Stable disease | 4 (44) | 3 (30) | 9 (45) |
| ≥12 wk | 2 (22) | 2 (20) | 4 (20) |
| Progressive disease | 4 (44) | 7 (70) | 9 (45) |
| Objective response rate, n (%)b | 1 (11) | 0 | 2 (10) |
| 95% CI | 0.3–48.2 | 0–30.8 | 1.2–31.7 |
Abbreviations: NIVO3, nivolumab 3 mg/kg; NIVO1+IPI3, nivolumab 1 mg/kg + ipilimumab 3 mg/kg; NIVO3+IPI1, nivolumab 3 mg/kg + ipilimumab 1 mg/kg. aBest overall response was assessed in response-evaluable patients per RANO criteria.30bRate of confirmed complete and partial responses.
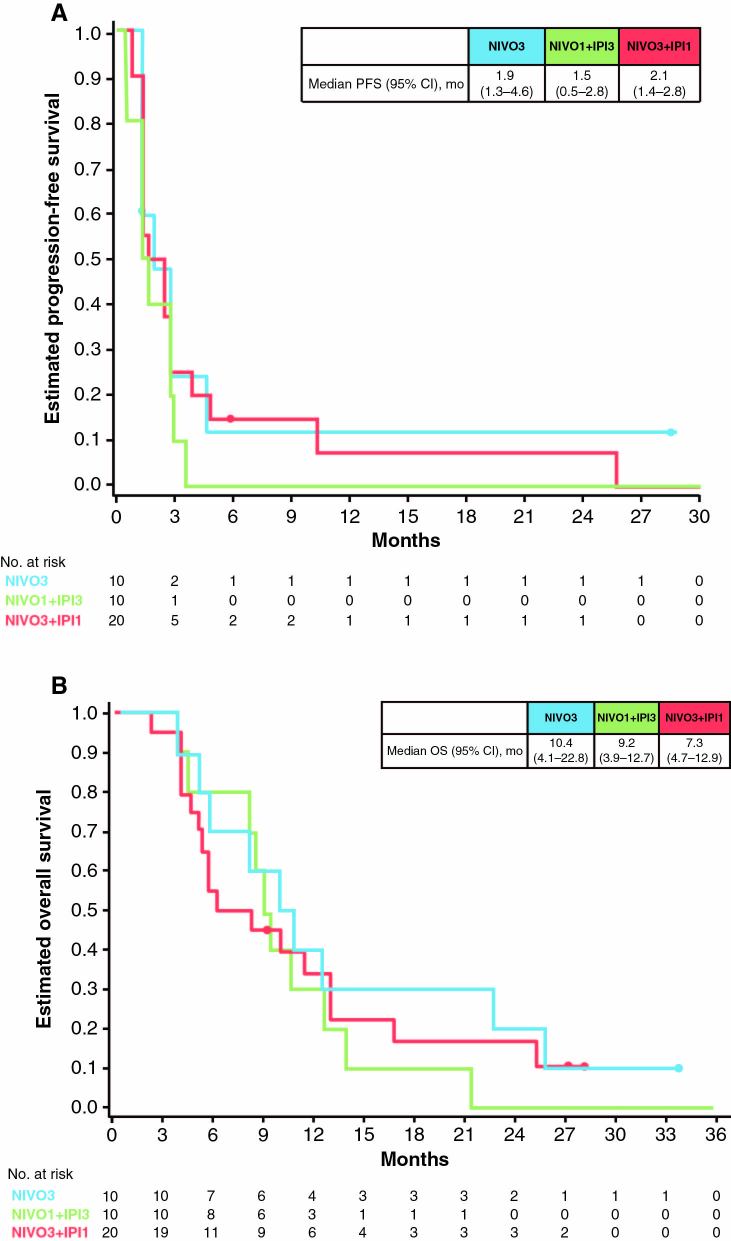
The median PFS (95% CI) among patients receiving NIVO3, NIVO1+IPI3, and NIVO3+IPI1 was 1.9 (1.3–4.6), 1.5 (0.5–2.8), and 2.1 months (1.4–2.8), respectively (Fig. 2A). Most patients (NIVO3, 90%; NIVO1+IPI3, 90%; NIVO3+IPI1, 75%) received subsequent therapy, including surgery, radiotherapy, and/or systemic therapy (Supplementary Table S3). Median (95% CI) OS was 10.4 (4.1–22.8), 9.2 (3.9–12.7), and 7.3 (4.7–12.9) months in patients treated with NIVO3, NIVO1+IPI3, and NIVO3+IPI1, respectively (Fig. 2B). Five patients (NIVO3, n = 2; NIVO3+IPI1, n = 3) survived >25 months, 3 of whom were still alive at the time of data cutoff (Supplementary Table S4). Of these long-term survivors, only 1 patient had a prolonged time from initial diagnosis to recurrence, the MGMT promoter status was methylated in 3 patients (all received NIVO3+IPI1), unmethylated in 1 (NIVO3), and not reported in 1 (NIVO3), and none were receiving steroids at baseline.
Fig. 2.
Kaplan–Meier estimates of (A) PFS and (B) OS for patients in the NIVO3, NIVO1+IPI3, and NIVO3+IPI1 treatment arms. Closed circles indicate patient censoring. Abbreviations: NE = not estimable.
Tumor PD-L1 Expression
Tumor-cell PD-L1 expression was assessed as an exploratory endpoint using tissue collected at the time of initial diagnosis in all patients except 1, for whom analyses were performed using tissue collected at the time of recurrence. Using immunohistochemistry, PD-L1 was evaluable in 37 of 40 patients overall (93%; Table 1). Among these patients (NIVO3, n = 10; NIVO1+IPI3, n = 9; NIVO3+IPI1, n = 18), 25 (68%) had PD-L1 expression levels ≥1% and 10 (27%) had PD-L1 expression levels ≥10%.
Treatment Effects
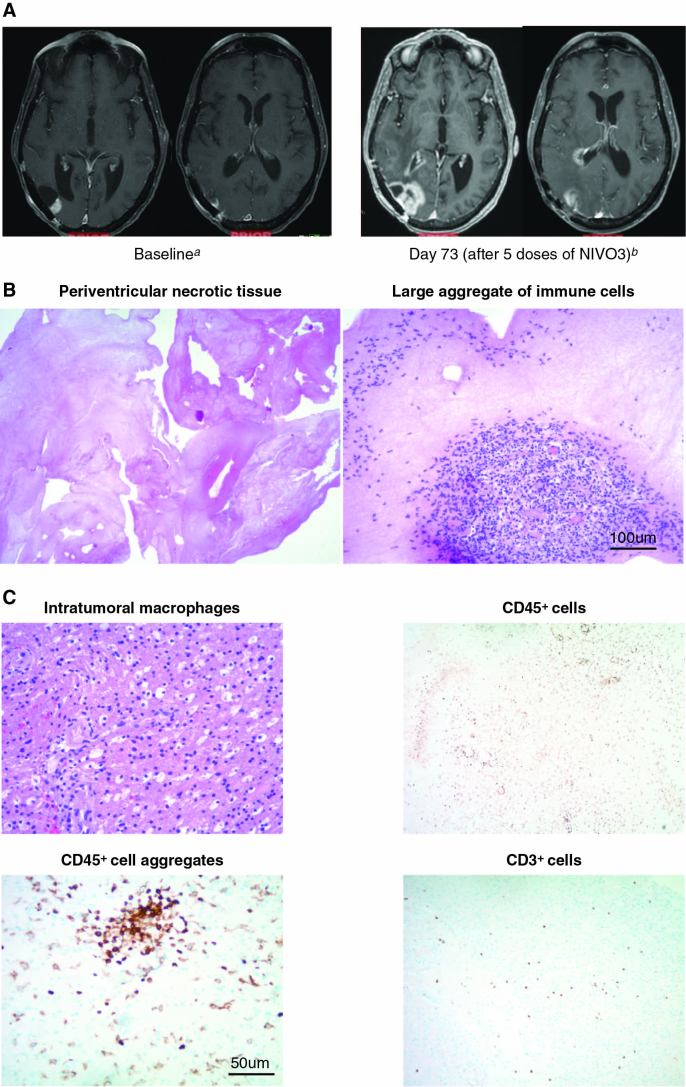
In some cases, immune checkpoint inhibitor therapy resulted in new areas of enhancement that were subsequently deemed not to be tumor outgrowth by central neuropathological review.34 Two patients who received NIVO3 and were initially identified as progressors were subsequently determined to be experiencing treatment effects instead of tumor outgrowth. One patient, a 67-year-old woman with an initial diagnosis of a lesion in the right temporal lobe, underwent gross total resection followed by radiotherapy concurrent with temozolomide and experienced recurrence less than 1 year after diagnosis. An MRI scan after 5 doses of NIVO3 (day 73) showed increased contrast enhancement of the temporal lobe lesion and new periventricular contrast enhancement relative to baseline (Fig. 3A), suggestive of disease progression. Subsequent histopathologic analysis of the resected tumor revealed large aggregates of immune cells, and no viable tumor was observed in the periventricular areas (Fig. 3B). Immune infiltrates consisted of macrophages, T cells, and other CD45+ cells, including CD45+ cell aggregates (Fig. 3C). The patient later died (~11 mo after initiating nivolumab therapy) of distant disease progression in the hippocampus and right hypothalamus.
Fig. 3.
A patient case depicting immune-mediated effects of therapy is presented. (A) MRI scans from a 67-year-old patient treated with NIVO3 who had suspected disease progression, with an increase in lesion size from 12 mm at baseline (left) to 40 mm at day 73 (right). MRI scans were conducted using the same parameters for each scan. (B) Resected tumor at day 81 stained with hematoxylin and eosin indicating immune-mediated changes in lesion size consistent with large aggregates of immune cells (right) and extensive tumor necrosis (left). Scale bar denotes 100 μm. (C) Immunohistochemistry of resected tumor specimens depicts infiltrating immune cell aggregates, T cells, and macrophages. Scale bar denotes 50 μm. a12-mm temporal lobe lesion; no corticosteroid treatment. b40-mm temporal lobe lesion; patient received concomitant methylprednisolone 16 mg/day.
Discussion
In this first article reporting on a prospective clinical trial of immune checkpoint inhibitors in patients with GBM, a population with a poor prognosis and few treatment options, nivolumab monotherapy was better tolerated than nivolumab in combination with ipilimumab and was selected for the phase III cohort (cohort 2) of CheckMate 143. The study presented here also found that tumor-cell PD-L1 expression was common in GBM tissue and there was no association detected between baseline steroid use and objective response with nivolumab ± ipilimumab. In addition, the neuropathological assessment suggests that immune checkpoint inhibitors may enhance inflammatory infiltrates in some patients with CNS tumors. However, considering the limitations of an uncontrolled, retrospective, single-case analysis, it is unclear whether the results in this case are due solely to treatment effects or to the natural history and/or heterogeneity of the disease.
The AE profiles in patients receiving nivolumab or nivolumab + ipilimumab were consistent with those observed in other tumor types, with no new safety concerns reported.15,16,18–21,35 Treatment with nivolumab monotherapy was associated with lower toxicity than either combination dose, similar to what was reported in melanoma.32,35 Importantly, concerns about treatment-limiting neurotoxicity and encephalitis caused by increased inflammatory brain infiltrates were not realized.
The potential of GBM tumors to be immunogenic is supported by the presence of infiltrating immune cells, including T cells, both before and during treatment with immune checkpoint inhibitor regimens, including nivolumab with or without ipilimumab. Evidence suggests that patients with GBM who have pretreatment immune activity within tumor sites may have a more favorable response to immune checkpoint inhibition.36 However, the GBM microenvironment is also notable for potent immunosuppression,37 and most patients in this study had detectable levels of PD-L1 on tumor cells. PD-L1 expression within the tumor microenvironment is known to promote accumulation of regulatory T cells and other immunosuppressive cell types.2,38 An association between clinical activity and tumor PD-L1 expression was not observed; however, definitive conclusions are precluded due to the small number of patients and the dynamic nature of PD-L1 expression from the time of initial diagnosis (when most samples used for PD-L1 analyses in this study were collected) to recurrence (time of study enrollment).34 Further investigation into the predictive value of PD-L1 expression, as well as other biomarkers such as mutational burden and neoantigen landscapes, is needed in patients with GBM.
Evaluation of efficacy per RANO criteria in this study was exploratory and limited by the small sample sizes, the multiple treatment schedules used, and the lack of a control treatment arm; the immunotherapy RANO (iRANO) criteria were not endorsed until after the CheckMate 143 protocol was written and therefore not used in this study. However, signals of antitumor activity were observed in some patients, including in 3 patients treated with NIVO3 or NIVO3+IPI1 who achieved a partial response and ≈20% of patients in each treatment arm who had stable disease for ≥12 weeks. Notably, 5 patients survived >25 months; however, among the overall population, preliminary median OS data with nivolumab (NIVO3, 10.4 months) or nivolumab + ipilimumab (NIVO1+IPI3, 9.2 months; NIVO3+IPI1, 7.3 months) appear consistent with those historically observed with other therapies in recurrent GBM.7–10,39–42 In the recently reported randomized phase III cohort 2 of CheckMate 143, which compared nivolumab monotherapy with bevacizumab monotherapy in patients with recurrent GBM, the primary endpoint of superior OS with nivolumab was not met.43 Other immune checkpoint inhibitors have also been evaluated in patients with GBM or CNS tumors in small, single-center studies, including a retrospective chart review of 22 patients with recurrent CNS tumors (11 of which were GBM) who showed no clinical or histopathologic efficacy with pembrolizumab (PD-1 inhibitor) monotherapy.44 Taken together, these data suggest that additional studies are needed to determine the optimal disease setting, clinical characteristics, and biomarkers that will identify patients who are most likely to benefit from immunotherapies or immunotherapy-containing regimens. Large phase III studies are ongoing (NCT02667587, NCT02617589) investigating nivolumab in combination with radiotherapy ± temozolomide in patients with newly diagnosed GBM.
Currently available treatment options for patients with recurrent GBM have demonstrated limited efficacy, underscoring the high unmet need in this patient population.7–10,39–42 Based on these results demonstrating the safety and tolerability of nivolumab, with or without ipilimumab, and potential treatment-related immune activity in the brain, clinical trials investigating nivolumab combination regimens continue, with the goals of identifying a population of patients who will benefit from these therapies and informing the treatment paradigm for these difficult-to-treat patients.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This study was supported by Bristol-Myers Squibb.
Acknowledgments
We thank all patients and families, as well as the study teams involved in the study, for making this trial possible. Medical writing and editorial assistance were provided by Christopher Reina and Jeff Bergen from Chrysalis Medical Communications, Inc. (Hamilton, New Jersey, USA), funded by Bristol-Myers Squibb.
Conflict of interest statement. Antonio Omuro has served as an advisor for Bristol-Myers Squibb, AstraZeneca, Merck, Stemline, Inovio, Alexion, Oxigene, CarThera, Novartis, and Juno Therapeutics. Michael Lim received research funding from Bristol-Myers Squibb, Accuray, and Agenus; served as an advisor for Merck, Oncrus, Boston Biomedical, and Agenus; received honoraria from Regeneron; and has a patent for combination radiation and checkpoint inhibitors. Solmaz Sahebjam received research funding from Bristol-Myers Squibb, Merck, and Cortice Biosciences and served as an advisor for Bristol-Myers Squibb and Merck. Timothy Cloughesy has served as a consultant for Bristol-Myers Squibb, Pfizer, Tocagen, Roche, Novocure, Nektar, VBL, AbbVie, Upshire Smith, Notable Labs, Oxigene, NewGen, Agios, Cortice Biosciences, MedQia, PRoNai, Wellcome, Merck, Insys, Human Longevity, Sunovion, Boston Biomedical, Alexion, and Novogen. Shakti H. Ramkissoon received research funding from Bristol-Myers Squibb. Keith L. Ligon received research funding from Bristol-Myers Squibb. Robert Latek, Ricardo Zwirtes, Prashni Paliwal, and Christopher T. Harbison are employees and stockholders of Bristol-Myers Squibb. Lewis Strauss is an employee of Bristol-Myers Squibb. David A. Reardon received research funding from Bristol-Myers Squibb, Celldex Therapeutics, Incyte, and Midatech and served as an advisor for AbbVie, Amgen, Bristol-Myers Squibb, Cavion, Celldex, EMD Serono, Genentech/Roche, Inovio, Juno Pharmaceuticals, Merck, Midatech, Momenta Pharmaceuticals, Novartis, Novocure, Oxigene, Regeneron, Stemline Therapeutics, and Boston Biomedical. John H. Sampson received royalties from Celldex; received shares/equity from Istari Oncology; and has acted as a consultant for Bristol-Myers Squibb. Gordana Vlahovic, Joachim Baehring, and Alfredo Voloschin have nothing to disclose.
References
- 1. Ostrom QT, Gittleman H, Fulop J et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol. 2016;18(suppl 4):iv1–iv75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol. 2015;11(9):504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stupp R, Hegi ME, Mason WP et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 4. National Comprehensive Cancer Network. 2017. NCCN Clinical Practice Guidelines in Oncology. Central Nervous System Cancers. Version I 2017. Available at https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Accessed September 15, 2017.
- 5. Campos B, Olsen LR, Urup T, Poulsen HS. A comprehensive profile of recurrent glioblastoma. Oncogene. 2016;35(45):5819–5825. [DOI] [PubMed] [Google Scholar]
- 6. Stupp R, Wong ET, Kanner AA et al. . NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48(14):2192–2202. [DOI] [PubMed] [Google Scholar]
- 7. Brada M, Hoang-Xuan K, Rampling R et al. . Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol. 2001;12(2):259–266. [DOI] [PubMed] [Google Scholar]
- 8. Reardon DA, Nabors LB, Mason WP et al. ; BI 1200 36 Trial Group and the Canadian Brain Tumour Consortium Phase I/randomized phase II study of afatinib, an irreversible ErbB family blocker, with or without protracted temozolomide in adults with recurrent glioblastoma. Neuro Oncol. 2015;17(3):430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raizer JJ, Grimm S, Chamberlain MC et al. . A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer. 2010;116(22):5297–5305. [DOI] [PubMed] [Google Scholar]
- 10. Batchelor TT, Mulholland P, Neyns B et al. . Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yervoy (ipilimumab) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2017. [Google Scholar]
- 12. Opdivo (nivolumab) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2017. [Google Scholar]
- 13. Opdivo (nivolumab) [summary of product characteristics]. Anagni, Italy; Bristol-Myers Squibb S.r.l.; Uxbridge, UK: Bristol-Myers Squibb Pharma EEIG; 2017. [Google Scholar]
- 14. Ansell SM, Lesokhin AM, Borrello I et al. . PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Motzer RJ, Escudier B, McDermott DF et al. ; CheckMate 025 Investigators Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Robert C, Long GV, Brady B et al. . Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. [DOI] [PubMed] [Google Scholar]
- 17. Brahmer J, Reckamp KL, Baas P et al. . Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Borghaei H, Paz-Ares L, Horn L et al. . Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferris RL, Blumenschein G Jr, Fayette J et al. . Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Overman MJ, McDermott R, Leach JL et al. . Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharma P, Retz M, Siefker-Radtke A et al. . Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–322. [DOI] [PubMed] [Google Scholar]
- 22. Weber JS, D’Angelo SP, Minor D et al. . Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. [DOI] [PubMed] [Google Scholar]
- 23. Younes A, Santoro A, Shipp M et al. . Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17(9):1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reardon DA, Freeman G, Wu C et al. . Immunotherapy advances for glioblastoma. Neuro Oncol. 2014;16(11):1441–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berghoff AS, Kiesel B, Widhalm G et al. . Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015;17(8):1064–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nduom EK, Wei J, Yaghi NK et al. . PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. 2016;18(2):195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reardon DA, Gokhale PC, Klein SR et al. . Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol Res. 2016;4(2):124–135. [DOI] [PubMed] [Google Scholar]
- 28. Margolin K, Ernstoff MS, Hamid O et al. . Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459–465. [DOI] [PubMed] [Google Scholar]
- 29. Queirolo P, Spagnolo F, Ascierto PA et al. . Efficacy and safety of ipilimumab in patients with advanced melanoma and brain metastases. J Neurooncol. 2014;118(1):109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Louis DN, Ohgaki H, Wiestler OD et al. . The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wen PY, Macdonald DR, Reardon DA et al. . Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology Working Group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 32. Wolchok JD, Kluger H, Callahan MK et al. . Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. US Department of Health and Human Services. National Cancer Institute. (2009) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 Available at https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed July 1, 2017.
- 34. Sahebjam S, Ramkissoon S, Baehring J et al. . Histopathologic review of suspected disease progression in patients with recurrent glioblastoma (GBM) receiving nivolumab ± ipilimumab: CheckMate 143 [abstract]. J Clin Oncol. 2017;35:2001. [Google Scholar]
- 35. Larkin J, Chiarion-Sileni V, Gonzalez R et al. . Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Han S, Zhang C, Li Q et al. . Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer. 2014;110(10):2560–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Antonios JP, Soto H, Everson RG et al. . Immunosuppressive tumor-infiltrating myeloid cells mediate adaptive immune resistance via a PD-1/PD-L1 mechanism in glioblastoma. Neuro Oncol. 2017;19(6):796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fecci PE, Mitchell DA, Whitesides JF et al. . Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(6):3294–3302. [DOI] [PubMed] [Google Scholar]
- 39. Nagane M, Nishikawa R, Narita Y et al. . Phase II study of single-agent bevacizumab in Japanese patients with recurrent malignant glioma. Jpn J Clin Oncol. 2012;42(10):887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Desjardins A, Reardon DA, Coan A et al. . Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer. 2012;118(5):1302–1312. [DOI] [PubMed] [Google Scholar]
- 41. Sepúlveda JM, Belda-Iniesta C, Gil-Gil M et al. . A phase II study of feasibility and toxicity of bevacizumab in combination with temozolomide in patients with recurrent glioblastoma. Clin Transl Oncol. 2015;17(9):743–750. [DOI] [PubMed] [Google Scholar]
- 42. Wick W, Puduvalli VK, Chamberlain MC et al. . Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28(7):1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reardon D, Omuro A, Brandes A et al. . Randomized phase 3 study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma: CheckMate 143. Neuro Oncol. 2017;19(suppl 3):iii21. [Google Scholar]
- 44. Blumenthal DT, Yalon M, Vainer GW et al. . Pembrolizumab: first experience with recurrent primary central nervous system (CNS) tumors. J Neurooncol. 2016;129(3):453–460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.