Abstract
Background
Memantine has shown clinical utility in preventing radiation-induced cognitive impairment, but the mechanisms underlying its protective effects remain unknown. We hypothesized that abnormal glutamate signaling causes radiation-induced abnormalities in neuronal structure and that memantine prevents synaptic toxicity.
Methods
Hippocampal cultures expressing enhanced green fluorescent protein were irradiated or sham-treated and their dendritic spine morphology assessed at acute (minutes) and later (days) times using high-resolution confocal microscopy. Excitatory synapses, defined by co-localization of the pre- and postsynaptic markers vesicular glutamate transporter 1 and postsynaptic density protein 95, were also analyzed. Neurons were pretreated with vehicle, the N-methyl-d-aspartate–type glutamate receptor antagonist memantine, or the glutamate scavenger glutamate pyruvate transaminase to assess glutamate signaling. For animal studies, Thy-1-YFP mice were treated with whole-brain radiotherapy or sham with or without memantine.
Results
Unlike previously reported long-term losses of dendritic spines, we found that the acute response to radiation is an initial increase in spines and excitatory synapses followed by a decrease in spine/synapse density with altered spine dynamics. Memantine pre-administration prevented this radiation-induced synaptic remodeling.
Conclusion
These results demonstrate that radiation causes rapid, dynamic changes in synaptic structural plasticity, implicate abnormal glutamate signaling in cognitive dysfunction following brain irradiation, and describe a protective mechanism of memantine.
Keywords: memantine, radiation, synapse
Importance of the study
Radiation, used therapeutically to treat brain tumors, causes long-term cognitive deficits that negatively impact the quality of life for cancer survivors. While radiation is known to deplete replicative cells, little is known regarding the effects of radiation on surviving cells, such as neurons. Here, we demonstrate that radiation precipitates an unexpected, rapid proliferation of synapses, which transfer information between neurons. Radiation-induced synaptic proliferation precedes a marked synaptic loss, and glutamate antagonists, including memantine, block both effects. This describes both a novel mechanism of radiation-induced brain dysfunction as well as a protective mechanism for memantine, which has shown clinical promise. Furthermore, the results suggest that prevention of abnormal glutamate signaling may have radioprotective effects if administered before radiation, whereas drugs that support synaptogenesis may be useful in the treatment of survivors already suffering from radiation-induced cognitive deficits.
Radiation is an essential therapy for both primary and metastatic central nervous system tumors. Although effective, brain radiotherapy results in deficits in attention, learning, memory, and other cognitive domains lasting for years or even decades.1 Collectively, these deficits impair the quality of life for survivors.2,3 The pathogenesis of radiation-induced cognitive impairment is widely studied, but most studies have focused on damage to stem cells and neuronal progenitors at later time points.4 While damage to these cells contributes to radiation injury, only a small number of neuronal progenitors remain after birth, giving rise to a low number of neurons in 2 restricted brain regions.5,6 Moreover, the onset of some radiation-mediated cognitive symptoms may be too rapid to be accounted for by the disruption of late-born neurons, which take weeks to integrate into existing neural circuits.5 Thus, additional targets are required to fully understand the nature of radiation injury.
Neurons are classically considered radiation resistant owing to their postmitotic state.7 However, recent in vivo studies have documented radiation-induced decreases in dendritic spine density and changes in spine morphology in hippocampal neurons, including in regions devoid of neuronal progenitors.8–10 These changes were observed days to weeks after radiation and are important because dendritic spines are the main sites of excitatory synapses in the brain and play critical roles in information processing and memory storage. Generally, alterations in spine density and morphology reflect equivalent changes in excitatory synapse density and strength, respectively. Synapse connectivity and functionality are thought to underlie essentially all cognitive functions of the brain.11,12 Thus, any changes observed after radiation could indicate synapse and circuit abnormalities leading to the cognitive deficits described above.
Despite an incomplete understanding of the pathogenesis of radiotherapy-induced cognitive damage, clinical progress has been made in preventing or minimizing such impairments using agents approved for other disorders. Given the clinical similarities between radiotherapy-induced cognitive decline and vascular dementia, the Radiation Therapy Oncology Group conducted a randomized phase III clinical trial testing memantine for the prevention of cognitive decline after whole-brain radiotherapy.13 Memantine, an antagonist of N-methyl-d-aspartate (NMDA)–type glutamate receptors (NMDARs), modulates neuronal transmission and synaptic plasticity and is approved for the treatment of Alzheimer’s disease by the US Food and Drug Administration. Patients receiving memantine concurrently with and after radiotherapy displayed cognitive outcomes superior to those of patients receiving placebo, even after memantine was discontinued.13 These results support the hypothesis that radiation-induced cognitive impairments involve glutamate and NMDAR-dependent mechanisms.
In the current study, we investigated the temporal dynamics of radiation effects on synaptic structure to test the hypothesis that radiation-induced alterations in dendritic structure are mediated by abnormal glutamate and NMDAR signaling. We determined that radiation elicits a rapid proliferation of hippocampal neuron dendritic spines and excitatory synapses, which precedes synaptic loss. By studying the effects of a glutamate scavenger and memantine on these phenomena, we show that radiation-induced spine and synaptic changes are likely mediated by NMDARs. This work offers novel insight into how radiation impacts neuron and brain function and opens new avenues for investigation and intervention.
Materials and Methods
Cell Culture and Transfection
All animal studies were approved by the Institutional Animal Care and Use Committees at Baylor College of Medicine and the University of Texas MD Anderson Cancer Center. Hippocampi were dissected from embryonic day (E)18 Long-Evans rats, and neurons were dissociated and cultured as previously described.14,15 Cells were seeded at a density of 70000–125000 cells/well in 24-well plates onto glass slides treated with nitric acid and coated with laminin and poly-d-lysine. Cells were cultured in Neurobasal medium with B27 supplement (Invitrogen), 2 mM glutamine, and 100 U/mL penicillin/streptomycin in a humidified incubator with 5% CO2 at 37°C. Cells were transfected with enhanced green fluorescent protein (eGFP) at 6 days in vitro using the calcium phosphate method.16
Radiation and Pharmacological Treatments
After 21 days in vitro, cultures were placed in 1.5X artificial cerebrospinal fluid (in mM: 204 NaCl, 15 glucose, 3.75 KCl, 1.95 MgCl2, 3 CaCl2, 15 HEPES, pH 7.3), either irradiated to a dose of 10 Gy from a cesium source or sham-processed, and then immediately returned to their reserved conditioned culture medium. Neuronal cultures were pre-incubated with 25 µM memantine-HCl, 5 units/mL glutamate pyruvate transaminase (GPT), or phosphate-buffered saline (PBS) for 15 minutes before irradiation or sham irradiation. For animal studies, postnatal day (P) 21–23 Thy-1-YFP line H mice were pretreated with 5 mg/kg memantine or vehicle via gavage 24 h and 1 h before irradiation to 10 Gy to the brain delivered with a cesium source. One hour following irradiation or sham, mice were perfused with saline and 4% paraformaldehyde, and their brains harvested, sectioned, and processed for imaging.
Cell Biochemistry and Immunoblotting
After irradiation or sham treatment, cells were removed from glass slides by agitating in homogenization buffer (50 mM Tris-HCl [pH 8], 25% sucrose, 1 mM EDTA) and sonicated; samples were then subjected to polyacrylamide gel electrophoresis on 7.5% sodium dodecyl sulfate gels and then wet-transferred to polyvinylidene difluoride membranes. Antibodies to postsynaptic density protein 95 (PSD-95) (PhosphoSolutions) were used to probe membranes at a dilution of 1:1000 mixed in 1% bovine serum albumin. The blots were developed with femto-sensitive enhanced chemiluminescence substrate (Thermo Scientific), imaged with a Kodak Imager, and analyzed with Carestream software (Kodak). Anti–beta actin staining was used as a loading control.
Immunochemistry and Microscopy
For live images, neurons were visualized using an Apotome microscope (Zeiss) operating in epifluorescence mode with a 63x objective. The first 10 transfected neurons encountered in a rastral sweep across the dish were selected, with their positions within dishes stored in the automated stage. Single images were collected at each time point for speed and to minimize photobleaching.
For imaging dendritic spines and synapses in culture, neurons were fixed with 4% formaldehyde and washed in PBS, and neurons were probed in 0.13% gelatin, 0.1% Triton X-100, 150 mM NaCl, and 20 mM phosphate, pH 7.4, with anti–vesicular glutamate transporter 1 (VGLUT1) (Synaptic Systems) antibodies and anti–PSD-95 (Thermo Scientific) antibodies to identify pre- and postsynaptic sites, respectively. After 18 h, cells were washed and treated with goat anti-mouse-Cy3 and anti-rabbit-Cy5 immunoglobulin G for 1 h and then mounted using an aqueous mounting solution. Confocal z-stack images were obtained using a Leica TCS SP2 confocal microscope with a 63x oil-immersion objective (∆z = 0.45 µm). Neurons selected for imaging were those whose somata were closest to blindly predetermined sites on coverslips.
Coronal slices from treated animals were imaged (∆z = 0.45 µm) and averaged 3 times on an Apotome pseudoconfocal microscope (Zeiss) using a 63x objective. Dorsal (septal) hippocampus was imaged between bregma −1.60 and −2.20 mm. For dentate gyrus, regions were imaged from the suprapyramidal blade at the apex of the arch. The dendrites from carbonic anhydrase 1 (CA1) pyramidal neurons immediately dorsal to this region were also imaged. As above, all samples were blinded with respect to treatment throughout data collection and analysis.
Data Analysis
Dendritic spine morphology was analyzed in 3D reconstructed z-stacks of neurons from both cultured neurons and in vivo experiments using Imaris software (Bitplane). For cultured neurons, only dendritic spines on secondary and tertiary dendrites were included. At least 100 µm of dendritic length was analyzed per neuron. For in vivo preparations, 4 neurons per stack (2 stacks per animal) were selected from randomly predetermined locations in the stacks. Secondary and tertiary dendrites from granule neurons and secondary dendrites from pyramidal CA1 neurons were traced and their spines analyzed. For both culture and in vivo preparations, dendritic spines were considered projections from the dendrite with clear head formation, whereas filopodia lacked a head structure. Structures were classified as spinules if they had no apparent origin from the dendrite outside of an existing spine. Technically, they could also be filopodia sprouting near the base of the spine. Staining patterns for VGLUT1 and PSD-95 were analyzed with Imaris; synapses were identified by co-localization of VGLUT1 and PSD-95 on overlaid image stacks.
Statistical Analysis
Data are presented as mean ± standard error of the mean. Statistical analyses were done with Prism 6, Igor Pro (WaveMetrics), and Stata/MP 15.0. For biochemical assays, statistical significance was determined by using Student’s t-test with a post hoc Bonferroni correction to account for multiple comparisons. One-, 2-, or 3-factor ANOVA was used to compare imaging parameters, with Tukey’s test used for post hoc analysis. P-values for 2- and 3-factor ANOVAs from throughout the manuscript are presented in Supplementary Tables S1 and S2.
Results
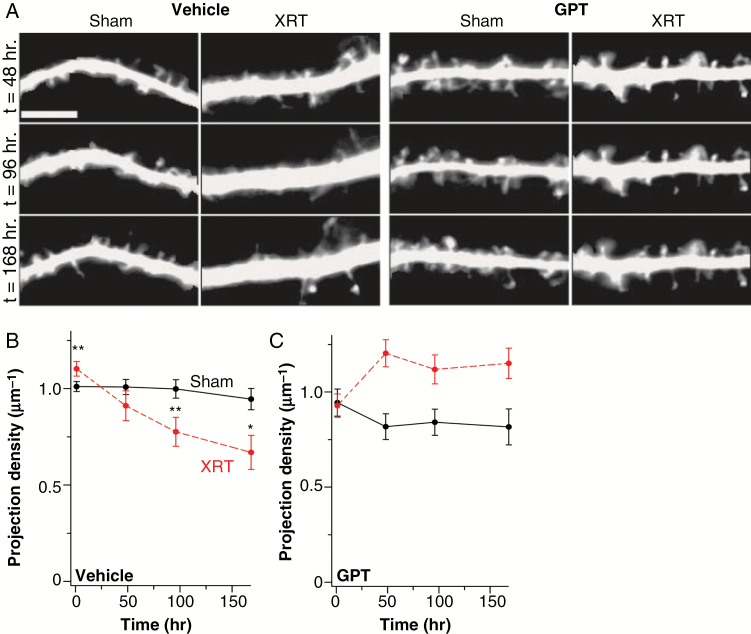
Recent studies report loss of dendritic spines in mature pyramidal neurons days to weeks after cranial irradiation of mice.9,10 We tested whether spine loss occurred in eGFP-expressing primary hippocampal cultures. Overall dendritic projection density (spines + filopodia) decreased in the cultures after radiation, reaching significantly lower levels 96 h post-irradiation (Fig. 1A, B). However, in assessing earlier time points, we detected an unexpected increase in dendritic projections 1 h after radiation (Fig. 1, 2). Such an increase in dendritic spines could reflect abnormalities in glutamate signaling. To investigate the potential role of abnormal glutamate signaling following radiation, we pretreated cultures with the glutamate scavenging enzyme GPT. Both the acute expansion and later loss of dendritic projections were prevented by GPT treatment (Fig. 1A, C, 2A–E). These observations suggest that radiation-induced loss of dendritic projections is preceded by an acute expansion of those projections and that both effects arise from altered glutamate signaling.
Fig. 1.
Irradiation causes long-term dendritic projection loss. (A) Representative longitudinal images of secondary dendrite segments from hippocampal neurons treated with vehicle or the glutamate scavenger GPT and subjected to sham treatment or irradiation (RT). Time after treatment is indicated on the left. (B, C) Summary data for experiments shown in panel A for vehicle- (B) or GPT-treated (C) neurons. Black lines and symbols represent data from sham-treated neurons, while red represents data from irradiated neurons. The statistical interaction between radiation, GPT, and time approached significance (Supplementary Table S2), while significance was achieved between different points (n = 8–39, N = 3). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. Bar is 5 µm.
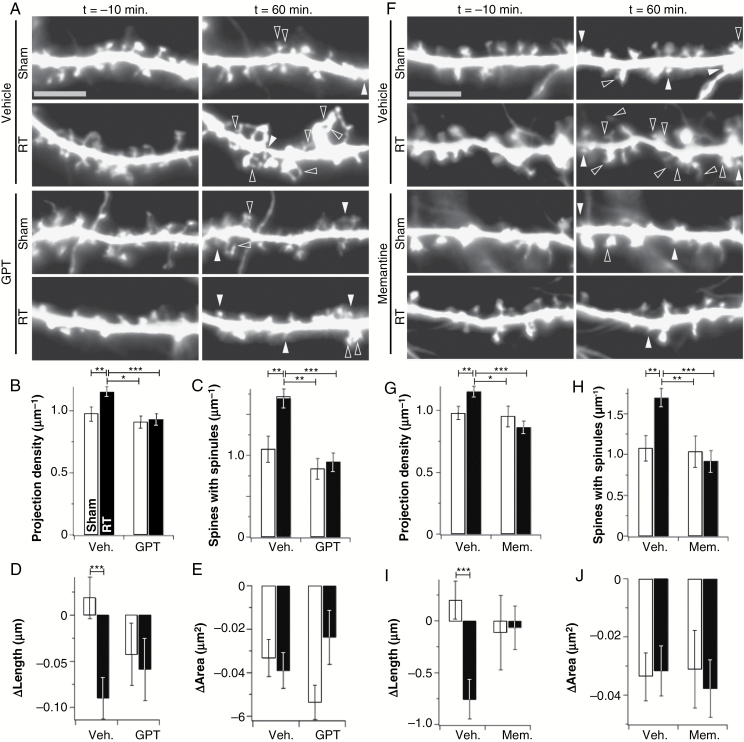
Fig. 2.
GPT and memantine prevent acute radiation-induced changes in live dendrite morphology. (A) Representative images of segments of secondary dendrites from vehicle- and GPT (5 units/mL)-treated neurons at the indicated time points after both sham treatment and irradiation (RT). Filled arrowheads indicate newly formed spines and open arrowheads indicate newly formed spinules and extra spine heads. GPT prevented increases in projection density (B) and spinules (C), as well as decreases in spine length (D). Data are shown as means ± SEM (n = 261–452 spines/13 neurons, N = 3). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. Bar is 5 µm. (F–J) The same experiment as in panels A–E was repeated using 25 µM memantine in place of GPT (n = 221–461 spines/13 neurons, N = 3). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. Bar is 5 µm.
Characterization of the acute aspects of neuronal injury following radiation exposure may provide insight into mechanisms underlying long-term damage. Moreover, because therapeutic radiation is administered in a planned setting, pretreatment with radioprotectors to prevent acute and thereby longer-term damage is easily done. We therefore examined aspects of acute injury in greater detail, using longitudinal imaging of hippocampal neurons. Radiation induced not only an increase in dendritic projection density, but also an increase in the density of spines with multiple heads and/or filopodia (spinules) emanating from their heads (Fig. 2A, C). Spines that persisted throughout the time course showed a small but significant decrease in length, whereas the same population in sham controls showed no change (Fig. 2A, D). Spine shortening often occurs during the maturation of a dendritic spine.17,18 GPT blocked these effects (Fig. 2A–E). Two-factor ANOVA confirmed that GPT altered the response of neurons to radiation (Supplementary Table S1).
We previously showed that irradiation of ex vivo hippocampal slices leads to NMDAR internalization and impaired synaptic plasticity.19 Morphological changes in dendritic spines are linked to NMDAR activity.14,20 Moreover, the NMDAR blocker memantine has clinical benefits in protecting cognition after radiotherapy.13 Therefore, we repeated the experiments in Fig. 2A–E using 25 µM memantine in place of GPT. Memantine was as effective as GPT in protecting against radiation-induced acute increases in dendritic projection density (Fig. 2F, G), spinule formation (Fig. 2F, H), and shortening of persistent spines (Fig. 2F, I). Radiation led to an approximately 2-fold increase in the rate of projection gain without affecting the rate of projection loss, and memantine blocked this increase in projection gain (Supplementary Figure S1). As above, statistics confirmed that memantine altered the response of neurons to radiation (Supplementary Table S1). Together, these findings indicate that the acute effects of radiation on dendritic structure are likely mediated by abnormal glutamate and NMDAR signaling, as scavenging glutamate and blocking NMDAR activity had the same protective effects.
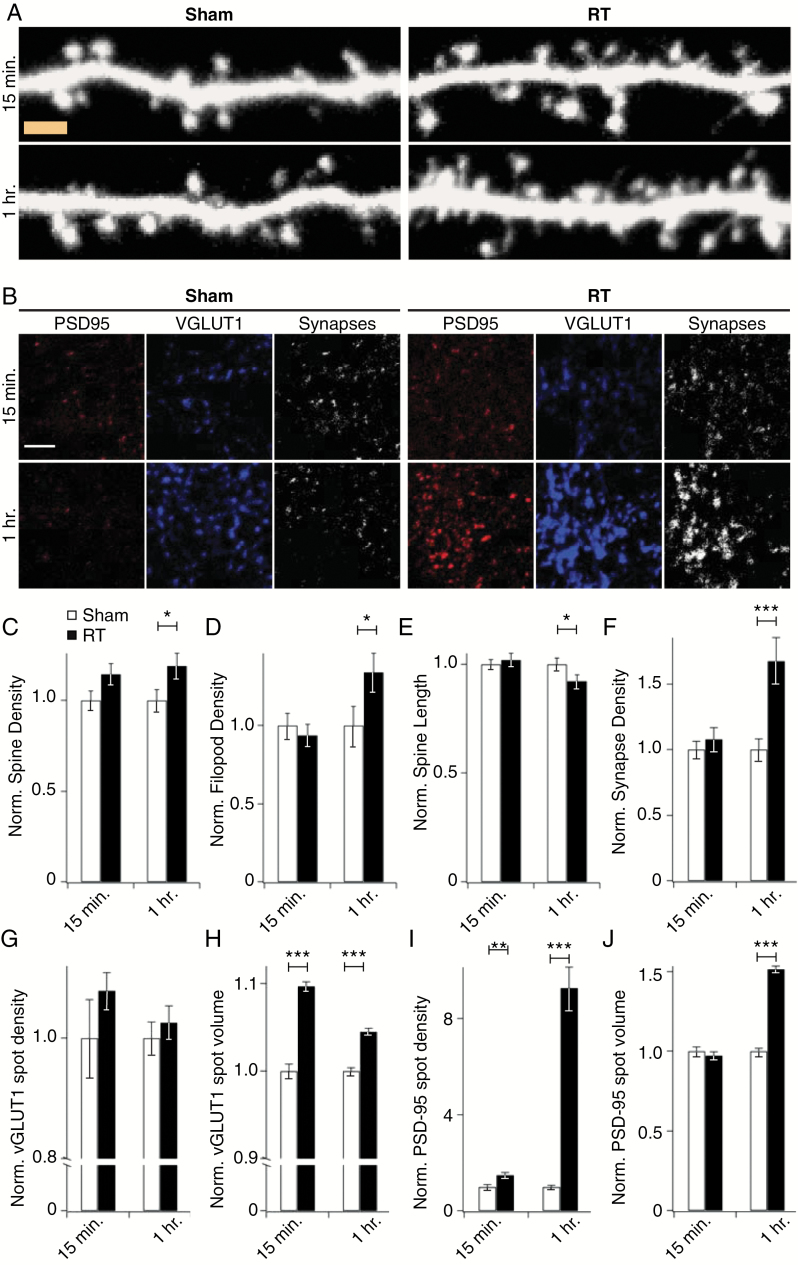
To complement these live-imaging studies and determine whether changes in dendritic architecture reflected synaptic changes, we subjected eGFP-expressing primary hippocampal neurons to irradiation or sham, fixed and processed them at various time points thereafter, and imaged dendrites by high-resolution confocal microscopy. This analysis revealed a 30% increase in density of spine and filopodia (precursors of spines) 1 hour after treatment relative to sham controls (Fig. 3A, C, D). As seen in Fig. 2, spine length shortened over the same time frame (Fig. 3A, E).
Fig. 3.
Radiation induces early alterations in dendritic and synaptic structure. Primary hippocampal neurons expressing eGFP were subjected to 10 Gy of radiation (RT) or sham and fixed and processed for high-resolution confocal microscopy at the indicated times. (A) Representative images reconstructed from confocal stacks at set time points after irradiation or sham treatment. Density of both spines (A, C) and filopods (A, D) increased and spine length decreased (A, E) in response to radiation. Data are shown as means ± SEM (for 15 min, n = 82 cells from 9 rats; for 1 hour, n = 100 cells from 10 rats). *P < 0.05. Bar is 2 µm. (B) Hippocampal neuron cultures received 10 Gy of RT or sham treatment and were fixed and stained for VGLUT1 and PSD-95 to identify pre- and postsynaptic compartments, respectively. Shown are representative images reconstructed from confocal stacks. Co-localization of the 2 markers revealed an increase in excitatory synapse density 1 h after RT (F). Analysis of VGLUT1 staining revealed no changes in density (G) and transient increases in the volume (H) of VGLUT1 puncta. Postsynaptic compartments exhibited a later response, with PSD-95 density (I) and volume (J) peaking significantly at 1 h after RT exposure. Staining data were obtained from non-overlapping fields with dimensions of 750 µm × 750 µm × 20 µm and the entire volume analyzed with Imaris. Data are shown as means ± SEM (for 15 min, n = 33 fields from 4 rats; for 1 h, n = 42 fields from 4 rats). **P ≤ 0.01, ***P ≤ 0.001. Bar is 5 µm.
Previous in vivo studies of radiation effects have shown convincing evidence of long-term pre- and postsynaptic structural changes days after radiation.10,21 To directly interrogate acute changes in the size and density of excitatory synapses, we co-stained for the postsynaptic marker PSD-95 and the presynaptic marker VGLUT1 and identified synaptic connections as defined by the overlap of these 2 markers (Fig. 3B). Synapse density increased after 1 h of radiation (Fig. 3B, F). We also assessed temporal alterations in pre- and postsynaptic components individually. At 15 min after exposure, presynaptic puncta (identified by VGLUT1 staining) had increased in density and volume (Fig. 3B, G, H). Postsynaptic changes followed a later and more pronounced pattern of change, with striking increases in the density and volume of postsynaptic puncta (identified by PSD-95 staining) (Fig. 3B, I, J) as well as the overall levels of PSD-95 (Supplementary Figure S2).
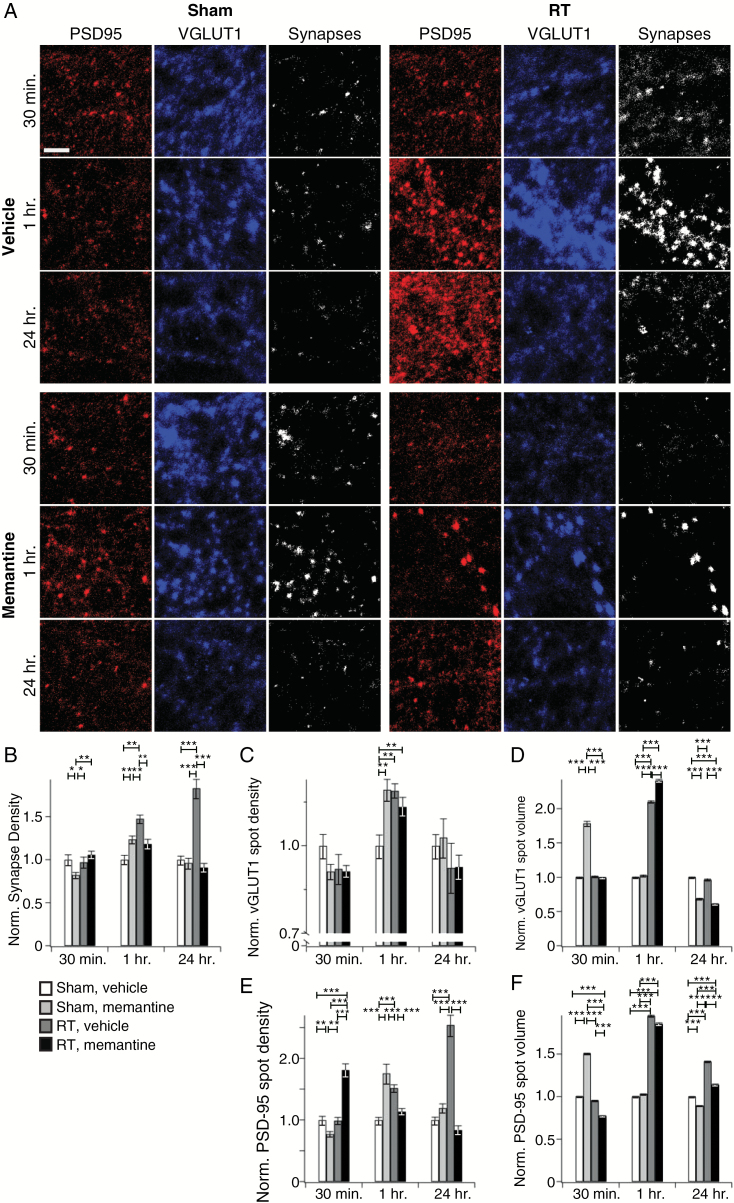
Given the protection afforded by memantine (Fig. 2F–J, Supplementary Table S1), we extended our high-resolution spine and synaptic analyses to include this drug. Memantine blocked radiation-induced increases in spine density, even when studies were extended to 24 h (Supplementary Figure S3A, B), and excitatory synapse density in memantine-treated neurons exhibited nearly complete protection from radiation-mediated increases at both 1 and 24 h after radiation (Fig. 4A, B). These apparently simple effects belie more complex dynamics, including: (i) Memantine alone caused the same transient increase in presynaptic punctal density as radiation, though these effects were not additive (Fig. 4A, C); (ii) memantine did not protect against the increase in presynaptic punctal volume 1 h after radiation (Fig. 4A, F); and (iii) memantine alone caused transient increases in postsynaptic punctal volume and density and failed to protect from or even exacerbated the effects of radiation on postsynaptic loci at 30 min and 1 h (Fig. 4A, E, F). Memantine altered neuronal responses to radiation with respect to synapse number and postsynaptic density and volume, but not with respect to presynaptic volume (Supplementary Table S1). Thus, while memantine had direct synaptic effects and did not protect against all aspects of radiation injury, the net effect was preservation of excitatory synapse and spine density.
Fig. 4.
Memantine prevents radiation-induced synaptic alterations. (A) Representative staining for presynaptic (VGLUT1)- and postsynaptic (PSD-95) markers and co-localization reconstructed from confocal stacks as a function of indicated time, sham vs radiation (RT), and drug treatment. (B) Analysis of VGLUT1/PSD-95 co-localization reveals that memantine prevented the previously identified increase in synaptic density at 1 h after RT. Presynaptic terminals, identified by VGLUT1 staining, demonstrated an increase in spot density (C) and volume (D) after RT or memantine alone. (E, F) Memantine had a strong protective effect on postsynaptic densities at 24 h but had more complex effects at shorter time points (see text for details). Data are shown as means ± SEM (n = 30–50 total neurons per condition per time point from 5 different animal preparations). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. Bar is 5 µm.
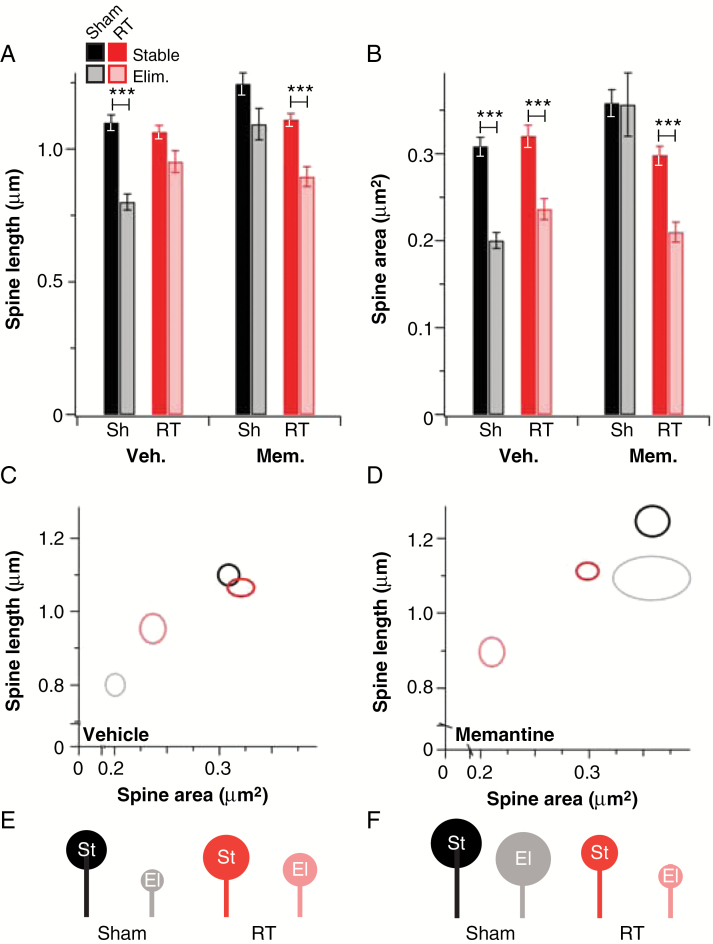
Having determined that radiation markedly and acutely affects steady-state spine and synapse levels, we took a deeper look at the effect of radiation on spine dynamics, since these give an indication of synapse dynamics.17,18 We initially determined overall rates of spine elimination and found that there was no change in spine elimination rates over the first hour after radiation relative to sham controls in the absence of memantine (Supplementary Figure S1). However, clear differences were apparent between the sizes of stable and eliminated spines in vehicle-/sham-treated neurons: Eliminated spines were ~25% shorter and had ~30% smaller heads on average than stable spines, suggesting that relatively weak synapses were preferentially eliminated. Irradiation erased this difference in length (Fig. 5A–D). Memantine treatment alone eliminated the differences in length and head size between stable and eliminated spines in sham-treated neurons, but these differences were completely restored when memantine-treated neurons were irradiated (Fig. 5A–D, Supplementary Table S2). These changes are depicted in cartoon form in Fig. 5E, F. Spines also formed within an hour of radiation, but radiation did not alter the length or cross-sectional area of these new spines relative to sham-treated controls (Supplementary Figure S4). Memantine alone led to increased length of newly formed spines, but concomitant radiation restored normal spine formation (Supplementary Figure S4). These results indicate that memantine and radiation alone each alter the rules of spine addition and elimination in hippocampal neurons, suggesting changes in synapse dynamics in each case. However, in combination, they restore at least the appearance of normal spine dynamics.
Fig. 5.
Features of stable and eliminated spines after acute irradiation. (A) Average initial length of stable (dark bars) and eliminated (light bars) spines in sham-treated (Sh, black bars) and irradiated (RT, red bars) neurons treated with vehicle or memantine. (B) Similar data for initial spine head area (n = 221–461 spines/13 neurons, N = 3). *P ≤ 0.05, ***P ≤ 0.001. Data in panel C show a 2-dimensional representation of spine length and area for sham-treated stable (black) and eliminated (gray) and RT-treated stable (red) and eliminated (pink) spines in vehicle-treated neurons. The same data for memantine-treated neurons are shown in panel D. Ovals are centered on the intersections of the values, and axes are representative of SEM. These data are represented in cartoon form for vehicle- (E) and memantine-treated (F) spines (st = stable, el = eliminated).
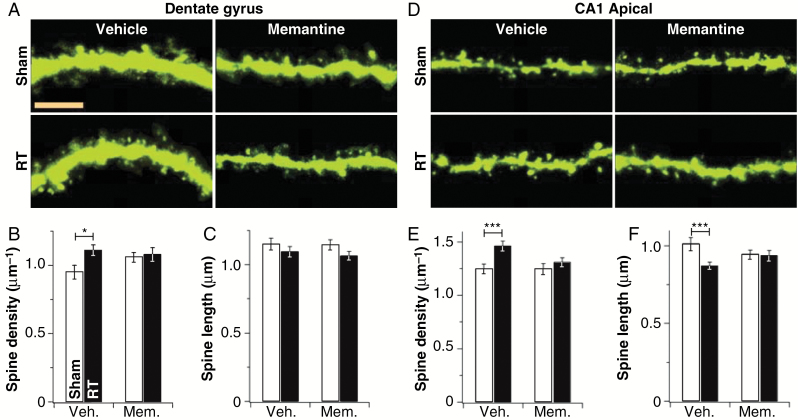
Finally, having carefully characterized acute radiation injury in hippocampal culture, we sought to determine if this injury occurs in vivo. To do so, we irradiated the brains of postnatal day (P) 21–23 Thy-1-YFP mice, which express yellow fluorescent protein (YFP) sparsely in a subset of hippocampal and cortical neurons, with a dose of 10 Gy.22 After 1 h, mice were perfused, their brains were removed and sectioned, and YFP-positive neurons in the dentate gyrus and CA1 regions of their hippocampi were imaged and analyzed for spine density and length. As in neuronal cultures, irradiation led to an increase in spine density in the dentate gyrus (Fig. 6A–C), apical CA1 (Fig. 6D–F), and basal CA1 (Supplementary Figure S5). Memantine treatment had no effect on baseline spine density in either dentate gyrus or apical CA1, but did prevent radiation-induced spine density increases (Fig. 6). The radiation-induced spine shortening observed in cultures (Fig. 1–3) was evident only in apical CA1 (Fig. 6D, E); this was also blocked by memantine. These results confirm that radiation-induced NMDAR-dependent acute spine expansion occurs in vivo. Further study of glutamate and synaptic dynamics in response to irradiation may lead not only to the use of agents with better protective capacity than memantine, but potentially ones which reverse abnormal dendritic structure in cancer survivors, potentially improving cognitive function.
Fig. 6.
Acute radiation injury in vivo. (A) Representative segments of granule cells from the suprapyramidal blade of Thy-1-YFP mouse dentate gyrus treated with sham or irradiated (RT) and vehicle or memantine treatment. Summary data for absolute spine density (B) and length (C) are also shown. Bar is 5 µm. (D–F) The same data as in panels A–C, but for proximal secondary apical dendrites of CA1 pyramidal neurons (N = 8 animals; 8 neurons per animal per region). *P ≤ 0.05, ***P ≤ 0.001.
Discussion
Here, we identified unexpected acute (~1 h) changes in neuronal morphology—specifically, rapid increases in dendritic spines and excitatory synapses—following radiation exposure. We also found evidence for the individual sensitivity of synapses to radiation and implicate abnormal glutamate signaling in radiation-induced synaptic alterations. Combined, these results offer a mechanism for the protective effects observed with memantine clinically and suggest new avenues for intervention.
Cognition arises from functional circuits comprising neurons linked by synaptic connections.11,12 Dendritic spines are the postsynaptic loci of most excitatory synapses in the brain. Thus, their density is a proxy for synapse density, and synapse gain and loss are mirrored by similar changes in spines.17,18 Spine morphology is a correlate for synaptic properties and spine head size positively correlates with synaptic strength.17,18 Both types of changes have functional implications, as they define the connectivity and gain properties of neural circuits.17,18 Here, we report that radiation increased spine formation rates over 1 h but did not change elimination rates (Supplementary Figure S1), resulting in increased spine density in hippocampal neurons (Fig. 1–3, 6, Supplementary Figures S3, S5). We verified the expected increase in synapses by testing for PSD-95/VGLUT1 overlap (Fig. 3, 4). We also saw evidence of circuit changes beyond the addition of spines and synapses: Radiation caused spinules to emerge from spine heads (Figure 2), typically evidence of circuit rewiring23,24; and whereas control neurons preferentially eliminated the expected short, small-headed spines, radiation suspended the rules of spine elimination, targeting larger spines less appropriate for acute elimination (Fig. 5). While memantine ameliorated many of these effects (Fig. 2, 4–6, Supplementary Figures S1, S3–S5), our data indicate that it has effects of its own and does not completely prevent early radiation injury (Fig. 4, 5, Supplementary Figures S4, S5).
Given the decreases in spines observed later than 100 h after radiation in culture (Fig. 1) and in vivo,8–10 acute increases in spines and synapses were unexpected. This acute radiation-induced circuit reorganization could contribute to later pathologies. In vivo studies of radiation effects have shown spine density and morphology to be decreased or altered both in the dentate gyrus and in the CA1 subregion of the hippocampus in mice weeks after 10 Gy radiation exposure to the brain.9 Similarly, Parihar and Limoli found that spine densities were reduced at 10 and 30 days after exposure, even at radiation doses as low as 1 Gy.25 In our later time points (>90 h), acute increases converted into projection losses (Fig. 1). Indeed, transient increases in spines and filopodia are in line with low-level glutamate toxicity, disorganized neuronal transmission, and several intellectual disabilities.26,27 As such, these early increases, potentially initiated by glutamate toxicity, likely represent a transition state on the way to synapse loss.
The mechanisms underlying acute radiation-induced changes in synaptic parameters are not clear. However, we found that GPT and memantine protected neurons from early changes, providing evidence that radiation has direct effects on synaptic communications between mature neurons and implicating abnormal glutamate/NMDAR signaling in the genesis of radiation injury. The source of abnormal glutamate and initiating mechanisms will require further study. We believe that the synaptic injury we describe is a distinct and novel effect of radiation and that, as in the case of other synaptic injuries, such as ischemic injury,28 prevention of the initial insult will block longer-term injuries, in contrast to the more classical nonsynaptic injuries associated with radiation.
Although much attention has been focused on deficits in neuronal precursors and other long-term sequelae of brain irradiation, our results highlight the acute effects of radiation on synaptic parameters and suggest a protective role for memantine in preserving dendritic spines and synapses. Clinically, memantine offers moderate protection from cognitive deficits after brain radiation,13 suggesting room for improvement. The practical consequences of our work are that knowledge of the acute radiation-induced synaptic injury may better inform additional strategies for therapeutic intervention. Radiation injury is unusual among brain injuries in that it is planned, rather than accidental, allowing interventions that are feasible and standardized before and immediately after treatment. Fixation on the ultimate outcome of radiation (ie, loss of dendritic spines [and, presumably, synapses]) could lead to the prescription of strategies that promote spine and synapse formation from the onset. Our results suggest that promoting spine and synapse formation could potentially increase the initial neuronal injury and ultimately exacerbate long-term radiation sequelae. Rather, our results recommend strategies to suppress spine and synapse formation and/or block glutamate signaling at the onset of injury and treatments to promote spine and synapse formation and stability only well after the radiation insult.
Further study of glutamate and synaptic dynamics in response to radiation may lead not only to the use of agents with better protective capacity than memantine but potentially ones which may reverse abnormal dendritic structure in cancer survivors previously treated with radiation to the brain, potentially improving cognitive function.
Supplementary Material
Supplementary material is available online at Neuro-Oncology (http://neuro-oncology.oxfordjournals.org/).
Funding
This work was supported by the American Cancer Society (IRG-08-061-01), the Cancer Prevention Research Institute of Texas (RP140430, NIH CA208535; to D.R.G.), NIH MH086119 and NIH K01MH086119 (to J.G.D.), and NIH NS062829 (to K.F.T.).
Acknowledgments
The authors thank Pamela Allen for her biostatical support. The authors also thank Katherine Zhu and Ethan Wang for assistance with experimental procedures, Yen-Kuei Tu and Kyongmi Um for assistance with neuronal cultures, Yen-Kuei Tu for assistance with data analysis, and Christine Wogan for her review of the manuscript.
Conflict of interest statement. The authors have no conflicts of interest to declare.
References
- 1. Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12(3):627–642. [DOI] [PubMed] [Google Scholar]
- 2. Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys. 1995;31(4):983–998. [DOI] [PubMed] [Google Scholar]
- 3. Jeffrey S. Wefel TAA, Kohli S. Neuropyschological function and quality of life. In: Andrew D, Norden DAR, Wen PY, eds. Primary Central Nervous System Tumors: Pathogeneis and Therapy. New York: Springer; 2010:143–160. [Google Scholar]
- 4. Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. [DOI] [PubMed] [Google Scholar]
- 5. Welzel G, Fleckenstein K, Schaefer J et al. . Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int J Radiat Oncol Biol Phys. 2008;72(5):1311–1318. [DOI] [PubMed] [Google Scholar]
- 6. Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124(3):319–335. [DOI] [PubMed] [Google Scholar]
- 7. Puck TT, Marcus PI. Action of X-rays on mammalian cells. J Exp Med. 1956;103(5):653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shirai K, Mizui T, Suzuki Y et al. . X irradiation changes dendritic spine morphology and density through reduction of cytoskeletal proteins in mature neurons. Radiat Res. 2013;179(6):630–636. [DOI] [PubMed] [Google Scholar]
- 9. Chakraborti A, Allen A, Allen B, Rosi S, Fike JR. Cranial irradiation alters dendritic spine density and morphology in the hippocampus. PLoS One. 2012;7(7):e40844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parihar VK, Limoli CL. Cranial irradiation compromises neuronal architecture in the hippocampus. Proc Natl Acad Sci U S A. 2013;110(31):12822–12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gruart A, Leal-Campanario R, Lopez-Ramos JC, Delgado-Garcia JM. Functional basis of associative learning and its relationships with long-term potentiation evoked in the involved neural circuits: lessons from studies in behaving mammals. Neurobiol Learn Mem. 2015;124:3–18. [DOI] [PubMed] [Google Scholar]
- 12. Grossberg S. From brain synapses to systems for learning and memory: object recognition, spatial navigation, timed conditioning, and movement control. Brain Res. 2015;1621:270–293. [DOI] [PubMed] [Google Scholar]
- 13. Brown PD, Pugh S, Laack NN et al. ; Radiation Therapy Oncology Group (RTOG) Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tolias KF, Bikoff JB, Burette A et al. . The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron. 2005;45(4):525–538. [DOI] [PubMed] [Google Scholar]
- 15. Duman JG, Tzeng CP, Tu YK et al. . The adhesion-GPCR BAI1 regulates synaptogenesis by controlling the recruitment of the Par3/Tiam1 polarity complex to synaptic sites. J Neurosci. 2013;33(16):6964–6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xia Z, Dudek H, Miranti CK, Greenberg ME. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J Neurosci. 1996;16(17):5425–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16(1):95–101. [DOI] [PubMed] [Google Scholar]
- 18. Bosch M, Hayashi Y. Structural plasticity of dendritic spines. Curr Opin Neurobiol. 2012;22(3):383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu PH, Coultrap S, Pinnix C et al. . Radiation induces acute alterations in neuronal function. PLoS One. 2012;7(5):e37677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwechter B, Rosenmund C, Tolias KF. RasGRF2 Rac-GEF activity couples NMDA receptor calcium flux to enhanced synaptic transmission. Proc Natl Acad Sci U S A. 2013;110(35):14462–14467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parihar VK, Pasha J, Tran KK, Craver BM, Acharya MM, Limoli CL. Persistent changes in neuronal structure and synaptic plasticity caused by proton irradiation. Brain Struct Funct. 2015;220(2):1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Feng G, Mellor RH, Bernstein M et al. . Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28(1):41–51. [DOI] [PubMed] [Google Scholar]
- 23. Carlin RK, Siekevitz P. Plasticity in the central nervous system: do synapses divide?Proc Natl Acad Sci U S A. 1983;80(11):3517–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spacek J, Harris KM. Trans-endocytosis via spinules in adult rat hippocampus. J Neurosci. 2004;24(17):4233–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parihar VK, Limoli CL. Cranial irradiation compromises neuronal architecture in the hippocampus. Proc Natl Acad Sci U S A. 2013;110(31):12822–12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pan F, Aldridge GM, Greenough WT, Gan WB. Dendritic spine instability and insensitivity to modulation by sensory experience in a mouse model of fragile X syndrome. Proc Natl Acad Sci U S A. 2010;107(41):17768–17773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dictenberg JB, Swanger SA, Antar LN, Singer RH, Bassell GJ. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev Cell. 2008;14(6):926–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ruan YW, Lei Z, Fan Y, Zou B, Xu ZC. Diversity and fluctuation of spine morphology in CA1 pyramidal neurons after transient global ischemia. J Neurosci Res. 2009;87(1):61–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.