Abstract
Antiretroviral therapy (ART) has rendered HIV-1 infection a treatable illness, however ART is not curative due to the persistence of replication-competent, latent proviruses in long-lived resting T cells. Strategies that target these latently infected cells and allow for immune recognition and clearance of this reservoir will be necessary to eradicate HIV-1 in infected individuals. This review describes current pharmacologic approaches to reactivate the latent reservoir in a manner that infected cells can be recognized and targeted, with the ultimate goal of achieving an HIV-1 cure.
Keywords: HIV-1, viral latency, HIV-1 reservoir, shock and kill, latency reversal agents, disulfiram, ingenol, benzotriazole, SMAC, NF-kappaB, protein kinase C, STAT5
Introduction
The introduction of combination antiretroviral therapy (ART) two decades ago turned the tide of the HIV-1 epidemic and represents a modern medical milestone. ART durably suppresses HIV-1 replication, allows for restoration of the immune system, prevents disease progression and reduces the risk of viral transmission to uninfected individuals. According to the World Health Organization, in 2015 roughly half of people living with HIV-1 infection (36.7 million individuals worldwide) have access to ART [http://www.unaids.org/en/resources/fact-sheet; accessed 10 May 2017]. Despite this remarkable success, over two million new HIV-1 infections occurred in 2015, and over one million deaths occurred due to AIDS [http://www.unaids.org/en/resources/fact-sheet; accessed 10 May 2017].
ART is not curative due to the nature of HIV-1 replication and viral tropism for CD4+ T cells. HIV-1 replication involves a proviral intermediate that is stably integrated into the cellular genome of activated CD4+ T cells. A minority of these cells revert to a quiescent state to persist for the life of the host as resting memory cells. The HIV-1 provirus persists in a reversibly quiescent state within these long-lived cells, and can efficiently initiate viral replication upon spontaneous or antigen-driven cellular activation(1–3). ART must be administered indefinitely in order to block new cycles of replication arising from this latently infected cellular reservoir when latent viruses reactivate(4; 5). The establishment of latency in resting memory CD4+ T cells likely occurs within days of primary HIV-1 infection(6). ART initiated early in the course of infection limits the size of the latent reservoir, but does not prevent its formation(7; 8). ART durably suppresses ongoing viral replication, however virus persists with minimal to no gene expression that would allow for immune recognition of cells harboring provirus. The latent reservoir in resting memory CD4+ T cells can be reliably quantified ex vivo in all patients on ART and does not appear to undergo significant decay in virally suppressed individuals on ART over a period of years(5; 9). Multiple clinical trials seeking to augment ART through the addition of antiretrovirals from complementary drug classes (ART intensification) have all demonstrated the same result: none of these trials reported alterations in low level viremia or reservoir size(10–12).
Life-long ART administration entails the risks of long-term drug toxicities as well as global strain on resources for a worldwide epidemic that continues to spread. Given the limitations of ART, evident at both a molecular and global scale, it has become clear that understanding and addressing viral persistence in patients on ART will be necessary to curb this epidemic. Research exploring the nature of HIV-1 persistence has accelerated, and has given rise to a wide variety of potential therapeutic strategies(13–18). One approach that has matured to the level of clinical trials is known as ‘shock and kill.’ In this strategy drugs are administered to patients on ART in order to induce proviral transcription in latently infected cells(19). This strategy is intended to make use of limited exposure to a pharmacologic agent to induce reservoir activation and/or depletion in a manner that would allow ART discontinuation without the risk of viral rebound. Arguably, this strategy may offer the possibility of a scalable solution to HIV-1 eradication. There have been over 15 completed clinical trials testing latency-reversing agents (LRAs) from distinct mechanistic classes(20; 21). However, only modest perturbation of the reservoir has been observed to date. This review aims to cover latency reversal agents that have shown promise at various stages of development in furthering this strategy a step closer towards the clinic.
HIV eradication strategies to date
The first clinical trials targeting the latent reservoir arose from the recognition that viral reactivation is concomitant with T cell activation(22; 23). Two small trials sought to activate T cells in vivo via co-administration of murine antibodies against human CD3 and interleukin-2 (IL-2)(24; 25). These interventions proved toxic to participants without demonstrating any obvious perturbation in the reservoir. These results influenced the field to avoid T cell activation in subsequent human trials. While more recent ‘shock and kill’ interventions have been well tolerated by participants, there has been little to no reservoir perturbation(20; 21). The modest results of trials so far, characterized by low-level transcriptional enhancements of latent proviruses without changes in latent reservoir size, have led to re-consideration of strategies relying on polyclonal T-cell activation.
Latency reversal agents
HIV-1 eradication trials have been largely informed by ‘hits’ from chemical compound screens performed in in vitro latency models. The modest efficacy of these compounds in human trials has led to re-evaluation of the predictive capacity of these models. A comparison of latency laboratory models evaluating the relative efficacy of known LRAs found wide heterogeneity(26). The only LRA class to demonstrate reproducible results across different latency models were the protein kinase C (PKC) agonists, compounds that induce robust T cell activation(26). A separate survey of LRAs representative of all actively studied classes revealed that the only consistently efficacious single agent in ex vivo experiments using patient cells was bryostatin-1, a PKC agonist(27; 28). Taken together, these results have led many in the field to reconsider the possibility that some degree of T cell activation may be required for efficient viral reactivation to occur. Latency reversal agents under active study can therefore be categorized into two groups: those that activate T cells in polyclonal fashion and those that do not (and instead use alternative pathways).
T cell activating agents
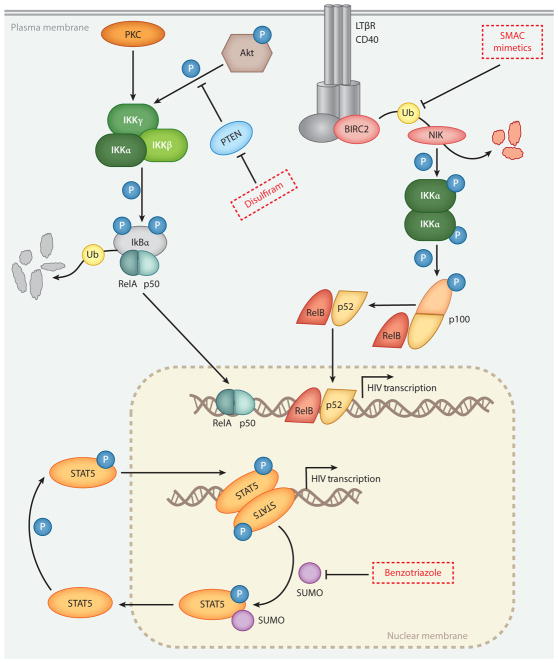
A subset of LRAs, the PKC agonists, activate T cells through a common mechanism involving stimulation of cellular protein kinase C isoforms, which in turn induce signaling via the transcription factor NF-κB. Protein kinase C enzymes are a family of serine/threonine kinases that engage in intracellular signal transduction. PKC enzymes are activated by the second messenger diacylglycerol (DAG)(29). PKC agonists act as mimics of DAG, binding to one or more cellular isoforms of protein kinase C to initiate downstream signaling. Activated PKC isoforms phosphorylate (and inactivate) IκB, which then releases RelA, the p65 sub-unit of NF-κB (Figure). NF-κB is free to enter the nucleus and bind to cognate binding sites in the viral LTR, inducing viral transcription. The role of targeting PKC-NF-κB signaling as a means to reactivate latent HIV-1 is shown in the Figure and has been reviewed in detail(29; 30). Among protein kinase C agonists, three chemical families are under active study: phorbol esters, including phorbol 12-myristate 13-acetate (PMA), prostratin and 12-deoxyphorbol 13-phenylacetate (DPP); macrocyclic lactones including bryostatin-1 and analogs; and diterpenes, which include ingenol compounds.
Figure. Signaling pathways leading to HIV-1 reactivation.
Signaling steps involved in viral reactivation by SMAC mimetics, Disulfiram and Benzotriazole derivatives. For details, see main text. Adapted from references 78 and 82.
Phorbol esters including PMA and prostratin are natural products that are potent PKC activators and reactivate provirus through NF-κB and AP-1 signal transduction(29). The tumor-promoting capacity of PMA has been well described in vitro (31), and has frequently been employed as a means to study oncogenesis(32). In the context of HIV-1 eradication, PMA is often used in vitro in combination with ionomycin as a strong inducer of T cell activation and latent proviral transcription(26; 28). However, PMA is not a candidate for clinical trials given its well-characterized oncogenic potential. Prostratin, a non-tumor-promoting phorbol ester with anti-viral properties, was first isolated from a poisonous plant, Pimela prostrata, native to New Zealand(33), and later identified in the medicinal plant Homalanthus nutans from Samoa(34). Prostratin induces proviral transcription in a variety of HIV-1 latency cell lines in vitro and also upregulates pro-inflammatory cytokines TNFα and IL-1β in vitro (35). Prostratin and DPP (12-deoxyphorbol 13-phenylacetate), both non-tumor-promoting phorbol esters, reverse latency in patient cells ex vivo and induce T cell activation(36; 37), though neither has been trialed in vivo.
Bryostatin-1 was isolated from a marine sponge and found to have anti-neoplastic activity in vitro (38; 39). Unlike PMA, bryostatin-1 is not tumor-promoting in vitro (39). Bryostatin-1 has been tested in multiple clinical trials against a variety of tumors(40). Myalgias are the most frequent adverse effect observed in vivo, and these correlate with elevated circulating levels of pro-inflammatory cytokines tumor necrosis factor α (TNFα) and interleukin 6 (IL-6)(41). One clinical trial has been conducted testing safety and efficacy of bryostatin-1 in the setting of HIV-1 eradication(42). Twelve participants were randomized to receive either placebo, a single infusion of bryostatin-1 at 10μg/m2 or a single infusion at 20μg/m2. No adverse events were recorded, however neither dose altered PKC activity above that of placebo. No changes in cell-associated viral RNA were observed.
Ingenols are diterpene compounds originally isolated from members of Euphorbia, a family of flowering plants. Euphorbia plants have been integral components of traditional medicine practices across many cultures for millennia (43). Ingenol 3,20 dibenzoate was isolated from Euphorbia esula and was identified to have anti-leukemic properties in vitro (44). With regard to HIV-1, ingenol compounds were first identified to antagonize viral replication via blocking viral adsorption(45). PKC agonists including ingenol-3-acetate, prostratin and PMA down-modulate CD4 and viral co-receptors CXCR4 and CCR5 on the cell surface(46). Semi-synthetic ingenols have been engineered in order to optimize their latency reversal activity(47; 48). Ingenol-3-mebutate (also known as ingenol-3-angelate) is FDA-approved as a topical therapy for actinic keratosis(49) and has demonstrated efficacy in multiple HIV-1 latency systems(46; 50). Ingenol B has recently been administered to non-human primates in combination with vorinostat (51). One of the two rhesus macaques exposed to ingenol B and vorinostat demonstrated increased SIV viral loads in both the central nervous system (CNS) and the periphery in response to LRA treatment, and also developed markers of systemic and CNS inflammation. A recent in vitro study of the effects of prostratin and bryostatin-1 on the central nervous system (CNS) identified that these agents may impair the integrity of the blood-brain barrier and increase trafficking of pro-inflammatory immune cells into the CNS(52), which may help to explain the results of the study by Gama and colleagues(51).
Side effects of T cell activating agents: mitigating pro-inflammatory cytokine responses
Given their potent T cell activation properties, valid concerns have been raised regarding systemic inflammation that could be triggered by these compounds(16; 27). In a phase I human in vivo dose-ranging study of bryostatin-1, plasma levels of pro-inflammatory cytokines TNFα and IL-6 were elevated within 2 hours of intravenous administration, and were temporally associated with dose-limiting toxicities among participants including severe myalgias(41). As mentioned above, the only clinical trial to date making use of a PKC agonist for HIV-1 eradication reported no adverse effects due to bryostatin-1(42). However, the investigators made use of doses of bryostatin-1 that did not achieve detectable systemic concentrations in a majority of trial participants, and did not induce latency reversal in any. One recently proposed alternative to address off-target PKC toxicity is to administer these compounds in their natural context. Euphorbia kansui has been used as part of traditional Chinese medicine as a cathartic and contains twelve ingenol compounds(53). Kansui plant extracts have been shown to reactivate HIV-1 in both cell lines in vitro and in patient cells ex vivo (54). Kansui is safe and has oral bioavailability as a tea. A pilot human trial of kansui via oral administration is planned (NCT02531295).
One plausible approach to minimize off-target effects of PKC activation would be to engineer PKC agonists that specifically target PKC isoforms responsible for proviral reactivation while minimally activating isoforms that induce pro-inflammatory cytokine release. Using DAG as a chemical scaffold, Hamer and colleagues synthesized a library of DAG lactones, and were able to identify several novel compounds that uncoupled latency reactivation from in vitro induction of TNFα using both the ACH-2 HIV-1 latency cell line and PBMCs from aviremic HIV-1 infected participants(55). A separate study generating novel phorbol-13-monoesters demonstrated differential effects on HIV-1 reactivation in a latently infected Jurkat T cell line(56). Latency reactivation efficacy in this study was correlated with the addition of increasingly lipophilic side chains to a phorbol core. Similarly, the ingenol core compound has no latency reversal activity in vitro, while the addition of lipophilic esters dramatically increases PKC activation and subsequent proviral transcription in vitro(48; 57).
Two groups have proposed a second possible approach to mitigate the off-target effects of T cell activation in the setting of PKC-induced HIV-1 latency reversal, which makes use of an adjuvant pharmacologic agent. Our laboratory recently demonstrated that the FDA-approved Janus kinase (JAK) inhibitor ruxolitinib significantly decreases pro-inflammatory cytokine release from resting CD4+ T cells and PBMCs from aviremic HIV-1 infected participants, but does not affect latency reversal induced by ingenol-3,20-dibenzoate(58). Martin and colleagues demonstrated that blocking MTORC1, a signaling complex that controls cellular metabolism and activation, using the FDA-approved immunosuppressant rapamycin (also known as sirolimus), could decrease cytokine release and cell proliferation in the setting of T cell activation via CD3 and CD28 antibodies(59). Using resting CD4+ T cells from aviremic HIV-1 positive donors, it was demonstrated that HIV-1 proviral reactivation was unaffected by the addition of rapamycin.
Furthermore, cytotoxic T lymphocyte (CTL) targeting of cells harboring reactivated provirus was unaffected by rapamycin in vitro. In an intriguing contrast to these results, recent work has demonstrated that simultaneous blockade of both MTORC1 and MTORC2 complexes by non-specific mTOR inhibitors significantly reduced HIV-1 latency reversal ex vivo (60). The role of mTOR signaling in the establishment and maintenance of HIV-1 latency clearly merits further study. Both ruxolitinib and rapamycin are the subject of pilot clinical trials that are actively enrolling aviremic HIV-1 positive participants at present (“Evaluating the Safety and Tolerability of Ruxolitinib in Antiretroviral-Treated HIV-Infected Adults,” NCT02475655; “Safety and Efficacy of Sirolimus for HIV Reservoir Reduction in Individuals on Suppressive ART,” NCT02440789). Although not designed to test ruxolitinib or sirolimus in combination with an LRA, these studies will provide crucial in vivo safety data to inform approaches making use of these adjuvant agents in combination with T cell-activating LRAs.
LRAs using alternative pathways
Initial HIV-1 eradication trials with OKT3 and IL-2 proved so profoundly toxic to patients (24; 25) that subsequent studies have only considered candidate LRAs with no T-cell activation properties(21). Several subsequent drug discovery efforts were based on the then uncertain premise that signals leading to latent HIV reactivation could be elicited in the absence of cellular activation, cellular proliferation and pro-inflammatory cytokine release. At the time, it was unclear that signaling pathways ‘alternative’ to canonical T-cell activation existed that would be capable of reactivating latent HIV. Nevertheless, more than a decade later this premise has been validated multiple times, as exemplified by the chemical hits discussed below. It is interesting that these drug-like molecules appear to trigger HIV transcription by activating unique, and often unexpected, pathways. The focus on ‘alternative’ pathways has produced a flurry of novel molecular targets that should keep medicinal chemists occupied for the foreseeable future. These novel hits appear to have an unfortunate common denominator, in that their potency is typically 5- to 10-fold less to that of T cell-activating regimens(26–28; 61).
HDAC inhibitors
Histone deacetylation is an important epigenetic modification contributing to proviral gene silencing(14), and inhibition of histone deacetylase enzymes has been demonstrated to reverse proviral latency in vivo in several clinical trials to varying degrees(62–66). HDAC inhibition has been the predominant mechanism of latency reversal in pilot clinical trials to date, and these agents have been generally well-tolerated by participants(21; 67). However, no trial employing HDAC inhibitors has demonstrated reservoir depletion to date. HDAC inhibitors have provided ‘proof-of-principle’ that latency can be safely perturbed in vivo(67), and these early trials serve as a foundation for a variety of combination therapies (discussed below and (20)). On the other hand, in vitro experiments using aviremic patient cells have demonstrated that viral reactivation, even when using potent regimens, occurs for only a minority of latently infected cells after a single administration of the LRA(61; 68).
Disulfiram
Disulfiram, an FDA-approved drug for alcohol cessation, was identified as a potential LRA using a primary cell model of latency(68). With regard to the mechanism of latency reversal, an in vitro study demonstrated that disulfiram depletes the intracellular protein PTEN, which in turn activates the Akt signaling pathway to initiate proviral transcription in an NF-κB-dependent manner (Figure)(69). Two clinical trials have tested disulfiram’s ability to perturb the latent reservoir in vivo (70; 71). The drug was well tolerated and modest increases in viral RNA were observed. Disulfiram has been evaluated in combination with other LRAs in vitro, however it did not demonstrate synergistic reactivation with PKC agonists or HDAC inhibitors(28).
Toll-like receptor agonists
Pathogen infections are primarily sensed by the innate immune system through the interaction of conserved molecular structures named pathogen-associated molecular patterns (PAMPs) with host-encoded pattern recognition receptors (PRRs) (72; 73). PRRs are germline-encoded receptors that recognize several classes of molecules typical of pathogens, such as proteins, lipids, carbohydrates and nuclei acids (73). Among PRRs, toll-like receptors (TLRs) have been widely studied. Flagellin, a potent stimulator of TLR-5, was shown to reactivate latent HIV-1 in quiescent central memory CD4+ T cells (74). In a later report, Pam3CSK4, a TLR-1/2 agonist, was shown to reactivate latent HIV-1 in an in vitro model of latency and in resting cells from aviremic patients (75). This reactivation is NF-κB, NFAT and AP-1-mediated and requires P-TEFb activity. This pathway differs from that initiated by T cell receptor engagement, which was shown to be mediated primarily by NFAT (76). Pam3CSK4-induced viral reactivation was achieved in the absence of T cell activation and proliferation. Therefore, the signaling pathway activated by Pam3CSK4 is highly selective for latent, integrated viruses and represents an attractive therapeutic target for eradication strategies.
Benzotriazole derivatives
A medium-throughput chemical screen using central memory CD4+ T cells and a GFP-tagged virus (76) identified 1-hydroxybenzotriazole (HOBt) as an active compound (77) and subsequently a number of active analogues have been identified. This family of compounds, benzotriazole derivatives, has no previously described biological function. Benzotriazoles reactivate latent HIV in primary cells and ex vivo in cells from aviremic patients, and do so in the absence of cellular activation, proliferation or toxicity(77). The most potent analogue identified so far is 3-Hydroxy-1,2,3-benzotriazin-4((3H)-one (HO-DHBt). HIV-1 reactivation by these compounds is dependent on STAT5 phosphorylation, which is initiated by γc-cytokine stimulation. The authors proposed that benzotriazoles inhibit a negative feedback loop mediated by covalent addition of SUMO2/3 to STAT5, which is required for its inactivation, dephosphorylation and export from the nucleus (78) (Figure). The result of combined benzotriazole and γc-cytokine stimulation is the sustained phosphorylation and activation of STAT5, for which cognate binding sites have been predicted in the viral promoter (79). Because of their mode of action, benzotriazoles have little to no activity in the absence of γc-cytokine stimulation(77).
SMAC mimetics and the non-canonical NF-κB activation pathway
The non-canonical NF-κB pathway is characterized by a slower onset, long-lasting transcriptional response, and higher functional selectivity, restricting its impact to a limited number of cellular processes and cell types (80). Activation of the non-canonical NF-κB pathway occurs through a specific subset of tumor necrosis factor receptors (TNFRs), including lymphotoxin beta receptor (LTβR) and CD40 (Figure). In complex with cIAP2, TRAF2, and TRAF3, cIAP1 constitutively degrades NF-κB-inducing kinase (NIK) thereby preventing p100 processing into p52. Inhibition of cIAP1 by SMAC mimetics leads to the accumulation of NIK, phosphorylation of IKKα, and the subsequent processing of p100 to p52. RelB/p52 heterodimers then translocate to the nucleus where they induce NF-κB-dependent transcription, including triggering transcription of latent proviruses (81). In vitro studies of HDAC inhibitor/SMAC/XIAP mimetic combinations demonstrated potent synergistic activities between these two classes of compounds(81).
LRA combination therapy
Combination approaches, in which LRAs from multiple mechanistic classes are administered simultaneously, hold promise for several reasons. Pilot clinical trials(21) as well as ex vivo experiments (28; 61) provide compelling evidence that no current single LRA appears able to reactivate a significant fraction of the latent reservoir. Furthermore, it remains unclear whether multiple doses of a single agent improve reservoir perturbation in vivo. In the case of the HDAC inhibitor vorinostat, cell-associated HIV-1 RNA increased approximately four-fold after a single administration to participants(62), however in a follow up study in which 22 doses of vorinostat were administered, minimal changes in cell-associated HIV-1 RNA were observed in vivo(82). Pharmacologic synergy, in which the activity of a LRA combination is greater than the sum of each LRA individually, could allow for smaller effective doses of each agent and, perhaps, lower frequency of administration. This in turn could maximize efficacy while minimizing potential side effects. Lastly, triggering multiple mechanisms at once may allow for a larger fraction of the reservoir to be reactivated after a single exposure.
With these goals in mind, several groups have explored potential LRA combinations in vitro [summarized in Table]. In a majority of these studies, PKC agonists have been combined with LRAs acting through alternative, non-T-cell activating mechanisms. Bryostatin-1 demonstrated synergistic reactivation when combined with the HDAC inhibitors panobinostat, romidepsin and vorinostat in latently infected cell lines (83) and ex vivo in resting CD4+ T cells(28). Bryostatin-1 also synergized with JQ1 in cell lines in vitro and patient cells ex vivo (28; 84). The PKC agonist prostratin has shown similar synergistic activity when combined with HDAC inhibitors(85), JQ1 (a BRD domain inhibitor) (28; 86), hexamethylene bisacetamide (HMBA, a P-TEFb release enhancer) (87) and a TLR-8 agonist (88). Ingenol B has demonstrated synergy with JQ1 in patient cells ex vivo(84). In separate work, ingenol-3-angelate showed similar synergistic behavior with JQ1 in patient cells ex vivo (50), however this result was not observed in a study that made use of resting CD4+ T cells from rhesus macaques(86). Several other studies have examined the combinatorial effect of novel LRAs. An inhibitor of transcriptional activator Runx1 called R05-3335 demonstrated synergy with vorinostat in patient PBMCs ex vivo (89). Panobinostat and romidepsin synergized with 5-azadC, a DNA demethylating agent (90). A human lectin, galectin-9, synergized in patient cells ex vivo with vorinostat and JQ1 (91). While it is difficult to directly compare results across these studies that make use of different in vitro and ex vivo systems and varying definitions of pharmacologic synergy, the overarching theme is that synergy appears to be achievable when combining different LRA classes. This is a promising observation that will hopefully translate into future LRA combination trials.
Table.
| LRA Combinations | ||||
|---|---|---|---|---|
| Reference | LRA 1 | LRA 2 | Model | Result |
| 85 | Prostratin | Vorinostat | JLat, U1, aviremic patient PBMCs (n=42) | apparent synergistic reactivation in patient PBMCs |
| Prostratin | TSA | |||
| 89 | Runx1 | Vorinostat | JLat, ACH2, TZMbl, aviremic patient PBMCs (n=6) | apparent synergistic reactivation in cell lines, low activity in patient PBMCs |
| 28 | Bryostatin-1 | Disulfiram | aviremic patient rCD4+ T cells (n=14) | synergy between Bryostatin-1 or Prostratin and JQ1 or HDACi; no synergy with disulfiram |
| Bryostatin-1 | JQ1 | |||
| Bryostatin-1 | Panobinostat | |||
| Bryostatin-1 | Romidepsin | |||
| Bryostatin-1 | Vorinostat | |||
| Prostratin | JQ1 | |||
| Prostratin | Romidepsin | |||
| Disulfiram | Panobinostat | |||
| Disulfiram | Romidepsin | |||
| Disulfiram | Vorinostat | |||
| Disulfiram | JQ1 | |||
| 50 | Ingenol-3-angelate | JQ1 | JLat, U1, aviremic patient rCD4+ T cells (n=13) | synergistic reactivation in patient CD4+ T cells |
| 84 | Ingenol B | JQ1 | JLat, U1, aviremic patient PBMCs (n=24) and rCD4+ T cells (n=15) | synergistic reactivation in all models |
| Bryostatin-1 | JQ1 | |||
| 83 | Bryostatin-1 | Panobinostat | J89GFP, THP89GFP cell lines | apparent synergistic reactivation in cell lines |
| Bryostatin-1 | Romidepsin | |||
| 90 | 5-azadC | Entinostat | JLat, aviremic patient PBMCs (n=24) | 5-azadC + panobinostat or romidepsin show apparent synergistic reactivation in patient cells |
| 5-azadC | Sodium butyrate | |||
| 5-azadC | Vorinostat | |||
| 5-azadC | Valproate | |||
| 5-azadC | Belinostat | |||
| 5-azadC | Panobinostat | |||
| 5-azadC | Romidepsin | |||
| 87 | Prostratin | HMBA | JLat, 2D10 cell lines | apparent synergistic reactivation in cell lines |
| 91 | Galectin-9 | JQ1 | JLat, aviremic patient rCD4+ T cells (n=13) | synergistic reactivation in patient CD4+ T cells for Gal-9 + SAHA and JQ1 |
| Galectin-9 | Panobinostat | |||
| Galectin-9 | Bryostatin-1 | |||
| Galectin-9 | Prostratin | |||
| Galectin-9 | Romidepsin | |||
| Galectin-9 | SAHA | |||
| 88 | TLR-8 agonist | Prostratin | JLat and MDDC co-culture, aviremic patient CD4 T cells (n=6) | apparent synergistic reactivation in cell lines, low activity in patient T cells |
| 86 | Ingenol-3-angelate | JQ1 | JLat, non-human primate CD4+ T cells (Rhesus macaques) | Ingenol-3-angelate reactivates latency at lower concentrations than other PKCa; no synergy observed with JQ1 |
| Prostratin | JQ1 | |||
| Ingenol-3-angelate | Prostratin | |||
| Ingenol-3-angelate | Bryostatin-1 | |||
Kill phase considerations
One of the fundamental conclusions from the first HIV-1 eradication trials testing the ‘shock and kill’ strategy is that the ‘kill’ phase, in which latently infected cells expressing viral RNA and antigens are targeted by the immune system or die of viral cytopathic effect, cannot be expected to passively follow the initial shock, at least with current LRAs(21; 67). A landmark set of experiments provided the biological basis for this lack of activity(92), and Jones and Walker have provided a thorough review of this topic(93). Several groups have investigated the effects that LRAs may have on effector cell function in vitro and as components of pilot HIV-1 eradication clinical trials. Jones and colleagues described that exposure to the HDAC inhibitors vorinostat, romidepsin or panobinostat lead to functional impairment of CD8+ T cells in vitro(94). Clutton and colleagues confirmed these findings and established the pharmacokinetics of these immune suppressive effects(95). Walker-Sperling and colleagues extended these observations and found that bryostatin-1 also had inhibitory effects on CD8+ T cell function(96), though interestingly they found that prostratin and JQ1 were not immune suppressive. A follow up study by the same group found that ingenol B also did not appear to affect CD8+ T cell function in vitro (97). The immunomodulatory effects of LRAs are not limited to CTL function. Two groups have characterized LRA-induced changes in both phenotype and function of NK cells(98; 99). PKC agonists induced generalized NK cell activation, while HDAC inhibitors decreased antiviral targeting by these innate immune cells.
Taken together, the results of these studies suggest that some form of immune boosting may be necessary for the ‘shock and kill’ strategy to succeed in the clinic. A recent clinical trial administering romidepsin in combination with therapeutic vaccine Vacc-4x and recombinant human granulocyte macrophage colony-stimulating factor (rhGM-CSF) demonstrated a non-significant decline in PCR-based measures of proviral DNA and was generally well-tolerated by participants(100). Additional trials testing romidepsin with a different vaccine candidate, MVA.HIVconsv (NCT02616874) and with the promising HIV-1 broadly neutralizing antibody 3BNC117 (NCT03041012) are actively recruiting participants at present. Several pilot clinical trials are in the recruitment or pre-enrollment phase that combine other LRAs with various means to stimulate antiviral immunity. Vorinostat will be administered with an HIV-1 vaccine candidate, ChAdV63.HIVconsv in two trials (NCT 01319383 and NCT02336074), with an innate immune stimulant AGS-004 (NCT02707900) and with hydroxychloroquine and maraviroc (NCT02475915). Panobinostat will be combined with pegylated interferon (NCT02471430). Current and upcoming clinical trials are summarized by Delagreverie et al. (20) and continuously updated on clinicaltrials.gov.
Durable suppression of latent proviral transcription
Efforts to pharmacologically reverse latency can be considered a means to attain a ‘sterilizing’ cure of HIV-1 through eradication of persistent viral reservoirs. An alternative approach to achieve ‘functional’ cure of HIV-1 infection (defined as long-term control of virus replication in the absence of ART; reviewed in (101; 102)) is the induction of long-lasting suppression of proviral gene expression. The Tat viral protein is a strong trans-activator of proviral gene expression, and its absence leads to severely restricted replication. Tat antagonists have been developed by several groups and appear to be potent inhibitors of HIV-1 transcription in vitro (103–105). Mousseau and colleagues have produced a novel Tat inhibitor, didehydro cortistatin A (dCA), with the unusual ability to induce a permanent state of latency that is present in culture many weeks after removal of the drug(105). Their in vitro experiments suggest that dCA binds to Tat’s basic domain, which mediates localization to the nucleolus and binding to the transactivation response element (TAR) (106). dCA thus prevents recruitment of Tat to the TAR stem/loop structure, presumably hindering the recruitment of P-TEFb and the onset of transcription elongation. Surprisingly, when treatment with dCA on latently infected HeLa-CD4 cells was interrupted, viral rebound was not observed for the remainder of the experiment (150 days), even after the addition of potent LRAs to the culture(105).
Rev is also essential for viral replication as it regulates the export of unspliced and singly spliced mRNAs. An inhibitor of Rev, ABX464, was recently identified, which had the ability to inhibit viral replication in culture as well as in humanized mice (107). When ART treatment was compared with ABX464 in humanized mice infected with HIV-1, it was noted that after viral suppression was achieved, removal of ART led to rebound in 6/6 mice. In contrast, removal of ABX464 led to rebound in only 2/6 animals, with viral loads in those two animals 200-fold lower than that in ART-treated animals. The protective effect lasted 52 days and was, therefore, equated to a functional cure.
Conclusions
Research addressing HIV-1 persistence is rapidly expanding, and distinct strategies are making their way to pilot clinical trials. It is hoped that the results of these trials will help establish consensus regarding the most efficacious approach to augment the suppressive effects of ART and curb the HIV-1 epidemic(108). Several critical issues remain to be answered in order for the field to advance, including gaining a better understanding of the contribution of non-T cell reservoirs to viral persistence (and means to target them)(109), and consensus definitions of the clinical and virologic correlates of LRA efficacy and reservoir perturbation in vivo(110). The diversity and creativity encompassed by current HIV-1 eradication approaches serves as a source of optimism that the resounding success of combination antiretroviral therapy two decades ago will be complemented someday soon by scalable clinical strategies to target the minute fraction of virus that persists despite ART.
Acknowledgments
Graphic design was provided by Xavier P. Matheson. This work was supported by NIH grants R01HL126547 (A.M.S. and V.P.), R21AI122377 (V.P.) and Doris Duke Charitable Foundation Clinical Scientist Development Award DDCF2016102 (A.M.S.). V.P. also receives support from NIH grants R01AI124843 (PI: Chanda) and UM1AI126620 (BEAT-HIV Delaney Collaborative to Cure HIV-1 Infection by Combination Immunotherapy), which is co-funded by NIAID, NIMH, NINDS, and NIDA.
References
- 1.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 1997;94:13193–7. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 3.Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–5. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 4.Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nature medicine. 1999;5:512–7. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 5.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nature medicine. 2003;9:727–8. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 6.Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014;512:74–7. doi: 10.1038/nature13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strain MC, Little SJ, Daar ES, Havlir DV, Gunthard HF, et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. The Journal of infectious diseases. 2005;191:1410–8. doi: 10.1086/428777. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson S, Graf EH, Dahl V, Strain MC, Yukl SA, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS pathogens. 2013;9:e1003174. doi: 10.1371/journal.ppat.1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crooks AM, Bateson R, Cope AB, Dahl NP, Griggs MK, et al. Precise Quantitation of the Latent HIV-1 Reservoir: Implications for Eradication Strategies. The Journal of infectious diseases. 2015;212:1361–5. doi: 10.1093/infdis/jiv218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinoso JB, Kim SY, Wiegand AM, Palmer SE, Gange SJ, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2009;106:9403–8. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandhi RT, Coombs RW, Chan ES, Bosch RJ, Zheng L, et al. No effect of raltegravir intensification on viral replication markers in the blood of HIV-1-infected patients receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;59:229–35. doi: 10.1097/QAI.0b013e31823fd1f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi RT, Zheng L, Bosch RJ, Chan ES, Margolis DM, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010:7. doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–7. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 14.Choudhary SK, Margolis DM. Curing HIV: Pharmacologic approaches to target HIV-1 latency. Annual review of pharmacology and toxicology. 2011;51:397–418. doi: 10.1146/annurev-pharmtox-010510-100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruelas DS, Greene WC. An integrated overview of HIV-1 latency. Cell. 2013;155:519–29. doi: 10.1016/j.cell.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xing S, Siliciano RF. Targeting HIV latency: pharmacologic strategies toward eradication. Drug discovery today. 2013;18:541–51. doi: 10.1016/j.drudis.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margolis DM. How Might We Cure HIV? Current infectious disease reports. 2014;16:392. doi: 10.1007/s11908-014-0392-2. [DOI] [PubMed] [Google Scholar]
- 18.Martin AR, Siliciano RF. Progress Toward HIV Eradication: Case Reports, Current Efforts, and the Challenges Associated with Cure. Annu Rev Med. 2016;67:215–28. doi: 10.1146/annurev-med-011514-023043. [DOI] [PubMed] [Google Scholar]
- 19.Deeks SG. HIV: Shock and kill. Nature. 2012;487:439–40. doi: 10.1038/487439a. [DOI] [PubMed] [Google Scholar]
- 20.Delagreverie HM, Delaugerre C, Lewin SR, Deeks SG, Li JZ. Ongoing Clinical Trials of Human Immunodeficiency Virus Latency-Reversing and Immunomodulatory Agents. Open Forum Infect Dis. 2016;3:ofw189. doi: 10.1093/ofid/ofw189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spivak AM, Planelles V. HIV-1 Eradication: Early Trials (and Tribulations) Trends Mol Med. 2016;22:10–27. doi: 10.1016/j.molmed.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A, Chen IS. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–22. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 23.Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–91. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 24.Kulkosky J, Nunnari G, Otero M, Calarota S, Dornadula G, et al. Intensification and stimulation therapy for human immunodeficiency virus type 1 reservoirs in infected persons receiving virally suppressive highly active antiretroviral therapy. The Journal of infectious diseases. 2002;186:1403–11. doi: 10.1086/344357. [DOI] [PubMed] [Google Scholar]
- 25.Prins JM, Jurriaans S, van Praag RM, Blaak H, van Rij R, et al. Immuno-activation with anti-CD3 and recombinant human IL-2 in HIV-1-infected patients on potent antiretroviral therapy. AIDS. 1999;13:2405–10. doi: 10.1097/00002030-199912030-00012. [DOI] [PubMed] [Google Scholar]
- 26.Spina CA, Anderson J, Archin NM, Bosque A, Chan J, et al. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS pathogens. 2013;9:e1003834. doi: 10.1371/journal.ppat.1003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nature medicine. 2014;20:425–9. doi: 10.1038/nm.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laird GM, Bullen CK, Rosenbloom DI, Martin AR, Hill AL, et al. Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. J Clin Invest. 2015;125:1901–12. doi: 10.1172/JCI80142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKernan LN, Momjian D, Kulkosky J. Protein Kinase C: One Pathway towards the Eradication of Latent HIV-1 Reservoirs. Adv Virol. 2012;2012:805347. doi: 10.1155/2012/805347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang G, Dandekar S. Targeting NF-kappaB Signaling with Protein Kinase C Agonists As an Emerging Strategy for Combating HIV Latency. AIDS research and human retroviruses. 2014 doi: 10.1089/aid.2014.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. The Journal of biological chemistry. 1982;257:7847–51. [PubMed] [Google Scholar]
- 32.Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984;308:693–8. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- 33.Zayed S, Hafez A, Adolf W, Hecker E. New tigliane and daphnane derivatives from Pimelea prostrata and Pimelea simplex. Experientia. 1977;33:1554–5. doi: 10.1007/BF01933991. [DOI] [PubMed] [Google Scholar]
- 34.Gustafson KR, Cardellina JH, 2nd, McMahon JB, Gulakowski RJ, Ishitoya J, et al. A nonpromoting phorbol from the samoan medicinal plant Homalanthus nutans inhibits cell killing by HIV-1. J Med Chem. 1992;35:1978–86. doi: 10.1021/jm00089a006. [DOI] [PubMed] [Google Scholar]
- 35.Kulkosky J, Culnan DM, Roman J, Dornadula G, Schnell M, et al. Prostratin: activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood. 2001;98:3006–15. doi: 10.1182/blood.v98.10.3006. [DOI] [PubMed] [Google Scholar]
- 36.Kulkosky J, Sullivan J, Xu Y, Souder E, Hamer DH, Pomerantz RJ. Expression of latent HAART-persistent HIV type 1 induced by novel cellular activating agents. AIDS research and human retroviruses. 2004;20:497–505. doi: 10.1089/088922204323087741. [DOI] [PubMed] [Google Scholar]
- 37.Williams SA, Chen LF, Kwon H, Fenard D, Bisgrove D, et al. Prostratin antagonizes HIV latency by activating NF-kappaB. The Journal of biological chemistry. 2004;279:42008–17. doi: 10.1074/jbc.M402124200. [DOI] [PubMed] [Google Scholar]
- 38.Pettit GR, Kamano Y, Fujii Y, Herald CL, Inoue M, et al. Marine animal biosynthetic constituents for cancer chemotherapy. J Nat Prod. 1981;44:482–5. doi: 10.1021/np50016a016. [DOI] [PubMed] [Google Scholar]
- 39.Smith JB, Smith L, Pettit GR. Bryostatins: potent, new mitogens that mimic phorbol ester tumor promoters. Biochem Biophys Res Commun. 1985;132:939–45. doi: 10.1016/0006-291x(85)91898-4. [DOI] [PubMed] [Google Scholar]
- 40.Kollar P, Rajchard J, Balounova Z, Pazourek J. Marine natural products: bryostatins in preclinical and clinical studies. Pharm Biol. 2014;52:237–42. doi: 10.3109/13880209.2013.804100. [DOI] [PubMed] [Google Scholar]
- 41.Philip PA, Rea D, Thavasu P, Carmichael J, Stuart NS, et al. Phase I study of bryostatin 1: assessment of interleukin 6 and tumor necrosis factor alpha induction in vivo. The Cancer Research Campaign Phase I Committee. J Natl Cancer Inst. 1993;85:1812–8. doi: 10.1093/jnci/85.22.1812. [DOI] [PubMed] [Google Scholar]
- 42.Gutierrez C, Serrano-Villar S, Madrid-Elena N, Perez-Elias MJ, Martin ME, et al. Bryostatin-1 for latent virus reactivation in HIV-infected patients on antiretroviral therapy. AIDS. 2016;30:1385–92. doi: 10.1097/QAD.0000000000001064. [DOI] [PubMed] [Google Scholar]
- 43.Ernst M, Grace OM, Saslis-Lagoudakis CH, Nilsson N, Simonsen HT, Ronsted N. Global medicinal uses of Euphorbia L. (Euphorbiaceae) J Ethnopharmacol. 2015;176:90–101. doi: 10.1016/j.jep.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 44.Kupchan SM, Uchida I, Branfman AR, Dailey RG, Jr, Fei BY. Antileukemic principles isolated from euphorbiaceae plants. Science. 1976;191:571–2. doi: 10.1126/science.1251193. [DOI] [PubMed] [Google Scholar]
- 45.Fujiwara M, Ijichi K, Tokuhisa K, Katsuura K, Shigeta S, et al. Mechanism of selective inhibition of human immunodeficiency virus by ingenol triacetate. Antimicrob Agents Chemother. 1996;40:271–3. doi: 10.1128/aac.40.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warrilow D, Gardner J, Darnell GA, Suhrbier A, Harrich D. HIV type 1 inhibition by protein kinase C modulatory compounds. AIDS research and human retroviruses. 2006;22:854–64. doi: 10.1089/aid.2006.22.854. [DOI] [PubMed] [Google Scholar]
- 47.Pandelo Jose D, Bartholomeeusen K, da Cunha RD, Abreu CM, Glinski J, et al. Reactivation of latent HIV-1 by new semi-synthetic ingenol esters. Virology. 2014;462–463:328–39. doi: 10.1016/j.virol.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abreu CM, Price SL, Shirk EN, Cunha RD, Pianowski LF, et al. Dual role of novel ingenol derivatives from Euphorbia tirucalli in HIV replication: inhibition of de novo infection and activation of viral LTR. PLoS One. 2014;9:e97257. doi: 10.1371/journal.pone.0097257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alchin DR. Ingenol mebutate: a succinct review of a succinct therapy. Dermatol Ther (Heidelb) 2014;4:157–64. doi: 10.1007/s13555-014-0061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang G, Mendes EA, Kaiser P, Wong DP, Tang Y, et al. Synergistic Reactivation of Latent HIV Expression by Ingenol-3-Angelate, PEP005, Targeted NF-kB Signaling in Combination with JQ1 Induced p-TEFb Activation. PLoS pathogens. 2015;11:e1005066. doi: 10.1371/journal.ppat.1005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gama L, Abreu CM, Shirk EN, Price SL, Li M, et al. Reactivation of simian immunodeficiency virus reservoirs in the brain of virally suppressed macaques. AIDS. 2017;31:5–14. doi: 10.1097/QAD.0000000000001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dental C, Proust A, Ouellet M, Barat C, Tremblay MJ. HIV-1 Latency-Reversing Agents Prostratin and Bryostatin-1 Induce Blood-Brain Barrier Disruption/Inflammation and Modulate Leukocyte Adhesion/Transmigration. J Immunol. 2017;198:1229–41. doi: 10.4049/jimmunol.1600742. [DOI] [PubMed] [Google Scholar]
- 53.Shi QW, Su XH, Kiyota H. Chemical and pharmacological research of the plants in genus Euphorbia. Chem Rev. 2008;108:4295–327. doi: 10.1021/cr078350s. [DOI] [PubMed] [Google Scholar]
- 54.Cary DC, Fujinaga K, Peterlin BM. Euphorbia Kansui Reactivates Latent HIV. PLoS One. 2016;11:e0168027. doi: 10.1371/journal.pone.0168027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamer DH, Bocklandt S, McHugh L, Chun TW, Blumberg PM, et al. Rational design of drugs that induce human immunodeficiency virus replication. J Virol. 2003;77:10227–36. doi: 10.1128/JVI.77.19.10227-10236.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marquez N, Calzado MA, Sanchez-Duffhues G, Perez M, Minassi A, et al. Differential effects of phorbol-13-monoesters on human immunodeficiency virus reactivation. Biochem Pharmacol. 2008;75:1370–80. doi: 10.1016/j.bcp.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Jiang G, Mendes EA, Kaiser P, Sankaran-Walters S, Tang Y, et al. Reactivation of HIV latency by a newly modified Ingenol derivative via protein kinase Cdelta-NF-kappaB signaling. AIDS. 2014;28:1555–66. doi: 10.1097/QAD.0000000000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spivak AM, Larragoite ET, Coletti ML, Macedo AB, Martins LJ, et al. Janus kinase inhibition suppresses PKC-induced cytokine release without affecting HIV-1 latency reversal ex vivo. Retrovirology. 2016;13:88. doi: 10.1186/s12977-016-0319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin AR, Pollack RA, Capoferri A, Ambinder RF, Durand CM, Siliciano RF. Rapamycin-mediated mTOR inhibition uncouples HIV-1 latency reversal from cytokine-associated toxicity. J Clin Invest. 2017;127:651–6. doi: 10.1172/JCI89552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Besnard E, Hakre S, Kampmann M, Lim HW, Hosmane NN, et al. The mTOR Complex Controls HIV Latency. Cell Host Microbe. 2016;20:785–97. doi: 10.1016/j.chom.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cillo AR, Sobolewski MD, Bosch RJ, Fyne E, Piatak M, Jr, et al. Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2014;111:7078–83. doi: 10.1073/pnas.1402873111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–5. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elliott JH, Wightman F, Solomon A, Ghneim K, Ahlers J, et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS pathogens. 2014;10:e1004473. doi: 10.1371/journal.ppat.1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV. 2014;1:e13–21. doi: 10.1016/S2352-3018(14)70014-1. [DOI] [PubMed] [Google Scholar]
- 65.Rasmussen TA, Tolstrup M, Moller HJ, Brinkmann CR, Olesen R, et al. Activation of latent human immunodeficiency virus by the histone deacetylase inhibitor panobinostat: a pilot study to assess effects on the central nervous system. Open Forum Infect Dis. 2015;2:ofv037. doi: 10.1093/ofid/ofv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sogaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, et al. The Depsipeptide Romidepsin Reverses HIV-1 Latency In Vivo. PLoS pathogens. 2015;11:e1005142. doi: 10.1371/journal.ppat.1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rasmussen TA, Tolstrup M, Sogaard OS. Reversal of Latency as Part of a Cure for HIV-1. Trends Microbiol. 2016;24:90–7. doi: 10.1016/j.tim.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 68.Xing S, Bullen CK, Shroff NS, Shan L, Yang HC, et al. Disulfiram reactivates latent HIV-1 in a Bcl-2-transduced primary CD4+ T cell model without inducing global T cell activation. Journal of virology. 2011;85:6060–4. doi: 10.1128/JVI.02033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doyon G, Zerbato J, Mellors JW, Sluis-Cremer N. Disulfiram reactivates latent HIV-1 expression through depletion of the phosphatase and tensin homolog. AIDS. 2013;27:F7–F11. doi: 10.1097/QAD.0b013e3283570620. [DOI] [PubMed] [Google Scholar]
- 70.Spivak AM, Andrade A, Eisele E, Hoh R, Bacchetti P, et al. A Pilot Study Assessing the Safety and Latency-Reversing Activity of Disulfiram in HIV-1-Infected Adults on Antiretroviral Therapy. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014;58:883–90. doi: 10.1093/cid/cit813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elliott JH, McMahon JH, Chang CC, Lee SA, Hartogensis W, et al. Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study. Lancet HIV. 2015;2:e520–9. doi: 10.1016/S2352-3018(15)00226-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Medzhitov R. TLR-mediated innate immune recognition. Semin Immunol. 2007;19:1–2. doi: 10.1016/j.smim.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 74.Thibault S, Imbeault M, Tardif MR, Tremblay MJ. TLR5 stimulation is sufficient to trigger reactivation of latent HIV-1 provirus in T lymphoid cells and activate virus gene expression in central memory CD4+ T cells. Virology. 2009;389:20–5. doi: 10.1016/j.virol.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 75.Novis CL, Archin NM, Buzon MJ, Verdin E, Round JL, et al. Reactivation of latent HIV-1 in central memory CD4(+) T cells through TLR-1/2 stimulation. Retrovirology. 2013;10:119. doi: 10.1186/1742-4690-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bosque A, Planelles V. Induction of HIV-1 latency and reactivation in primary memory CD4+ T cells. Blood. 2009;113:58–65. doi: 10.1182/blood-2008-07-168393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bosque A, Nilson KA, Macedo AB, Spivak AM, Archin NM, et al. Benzotriazoles Reactivate Latent HIV-1 through Inactivation of STAT5 SUMOylation. Cell Rep. 2017;18:1324–34. doi: 10.1016/j.celrep.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van Nguyen T, Angkasekwinai P, Dou H, Lin FM, Lu LS, et al. SUMO-specific protease 1 is critical for early lymphoid development through regulation of STAT5 activation. Mol Cell. 2012;45:210–21. doi: 10.1016/j.molcel.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Selliah N, Zhang M, DeSimone D, Kim H, Brunner M, et al. The gammac-cytokine regulated transcription factor, STAT5, increases HIV-1 production in primary CD4 T cells. Virology. 2006;344:283–91. doi: 10.1016/j.virol.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 80.Sun SC. Non-canonical NF-kappaB signaling pathway. Cell Res. 2011;21:71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pache L, Dutra MS, Spivak AM, Marlett JM, Murry JP, et al. BIRC2/cIAP1 Is a Negative Regulator of HIV-1 Transcription and Can Be Targeted by Smac Mimetics to Promote Reversal of Viral Latency. Cell Host Microbe. 2015;18:345–53. doi: 10.1016/j.chom.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Archin NM, Bateson R, Tripathy MK, Crooks AM, Yang KH, et al. HIV-1 expression within resting CD4+ T cells after multiple doses of vorinostat. The Journal of infectious diseases. 2014;210:728–35. doi: 10.1093/infdis/jiu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martinez-Bonet M, Clemente MI, Serramia MJ, Munoz E, Moreno S, Munoz-Fernandez MA. Synergistic Activation of Latent HIV-1 Expression by Novel Histone Deacetylase Inhibitors and Bryostatin-1. Sci Rep. 2015;5:16445. doi: 10.1038/srep16445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Darcis G, Kula A, Bouchat S, Fujinaga K, Corazza F, et al. An In-Depth Comparison of Latency-Reversing Agent Combinations in Various In Vitro and Ex Vivo HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression. PLoS pathogens. 2015;11:e1005063. doi: 10.1371/journal.ppat.1005063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reuse S, Calao M, Kabeya K, Guiguen A, Gatot JS, et al. Synergistic activation of HIV-1 expression by deacetylase inhibitors and prostratin: implications for treatment of latent infection. PLoS One. 2009;4:e6093. doi: 10.1371/journal.pone.0006093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brogdon J, Ziani W, Wang X, Veazey RS, Xu H. In vitro effects of the small-molecule protein kinase C agonists on HIV latency reactivation. Sci Rep. 2016;6:39032. doi: 10.1038/srep39032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen D, Wang H, Aweya JJ, Chen Y, Chen M, et al. HMBA Enhances Prostratin-Induced Activation of Latent HIV-1 via Suppressing the Expression of Negative Feedback Regulator A20/TNFAIP3 in NF-kappaB Signaling. Biomed Res Int. 2016;2016:5173205. doi: 10.1155/2016/5173205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rochat MA, Schlaepfer E, Speck RF. Promising Role of Toll-Like Receptor 8 Agonist in Concert with Prostratin for Activation of Silent HIV. J Virol. 2017:91. doi: 10.1128/JVI.02084-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Klase Z, Yedavalli VS, Houzet L, Perkins M, Maldarelli F, et al. Activation of HIV-1 from latent infection via synergy of RUNX1 inhibitor Ro5-3335 and SAHA. PLoS pathogens. 2014;10:e1003997. doi: 10.1371/journal.ppat.1003997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bouchat S, Delacourt N, Kula A, Darcis G, Van Driessche B, et al. Sequential treatment with 5-aza-2′-deoxycytidine and deacetylase inhibitors reactivates HIV-1. EMBO Mol Med. 2016;8:117–38. doi: 10.15252/emmm.201505557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abdel-Mohsen M, Chavez L, Tandon R, Chew GM, Deng X, et al. Human Galectin-9 Is a Potent Mediator of HIV Transcription and Reactivation. PLoS pathogens. 2016;12:e1005677. doi: 10.1371/journal.ppat.1005677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jones RB, Walker BD. HIV-specific CD8(+) T cells and HIV eradication. J Clin Invest. 2016;126:455–63. doi: 10.1172/JCI80566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jones RB, O’Connor R, Mueller S, Foley M, Szeto GL, et al. Histone deacetylase inhibitors impair the elimination of HIV-infected cells by cytotoxic T-lymphocytes. PLoS pathogens. 2014;10:e1004287. doi: 10.1371/journal.ppat.1004287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clutton G, Xu Y, Baldoni PL, Mollan KR, Kirchherr J, et al. The differential short- and long-term effects of HIV-1 latency-reversing agents on T cell function. Sci Rep. 2016;6:30749. doi: 10.1038/srep30749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walker-Sperling VE, Pohlmeyer CW, Tarwater PM, Blankson JN. The Effect of Latency Reversal Agents on Primary CD8+ T Cells: Implications for Shock and Kill Strategies for Human Immunodeficiency Virus Eradication. E Bio Medicine. 2016;8:217–29. doi: 10.1016/j.ebiom.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kwaa AK, Goldsborough K, Walker-Sperling VE, Pianowski LF, Gama L, Blankson JN. The effect of Ingenol-B on the suppressive capacity of elite suppressor HIV-specific CD8+ T cells. PLoS One. 2017;12:e0174516. doi: 10.1371/journal.pone.0174516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pace M, Williams J, Kurioka A, Gerry AB, Jakobsen B, et al. Histone Deacetylase Inhibitors Enhance CD4 T Cell Susceptibility to NK Cell Killing but Reduce NK Cell Function. PLoS pathogens. 2016;12:e1005782. doi: 10.1371/journal.ppat.1005782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Garrido C, Spivak AM, Soriano-Sarabia N, Checkley MA, Barker E, et al. HIV Latency-Reversing Agents Have Diverse Effects on Natural Killer Cell Function. Front Immunol. 2016;7:356. doi: 10.3389/fimmu.2016.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leth S, Schleimann MH, Nissen SK, Hojen JF, Olesen R, et al. Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): a single-arm, phase 1B/2A trial. Lancet HIV. 2016;3:e463–72. doi: 10.1016/S2352-3018(16)30055-8. [DOI] [PubMed] [Google Scholar]
- 101.Mylvaganam GH, Silvestri G, Amara RR. HIV therapeutic vaccines: moving towards a functional cure. Curr Opin Immunol. 2015;35:1–8. doi: 10.1016/j.coi.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eisele E, Siliciano RF. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity. 2012;37:377–88. doi: 10.1016/j.immuni.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jin H, Li D, Sivakumaran H, Lor M, Rustanti L, et al. Shutdown of HIV-1 Transcription in T Cells by Nullbasic, a Mutant Tat Protein. MBio. 2016:7. doi: 10.1128/mBio.00518-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cupelli LA, Hsu MC. The human immunodeficiency virus type 1 Tat antagonist, Ro 5-3335, predominantly inhibits transcription initiation from the viral promoter. J Virol. 1995;69:2640–3. doi: 10.1128/jvi.69.4.2640-2643.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mousseau G, Kessing CF, Fromentin R, Trautmann L, Chomont N, Valente ST. The Tat Inhibitor Didehydro-Cortistatin A Prevents HIV-1 Reactivation from Latency. MBio. 2015;6:e00465. doi: 10.1128/mBio.00465-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mousseau G, Clementz MA, Bakeman WN, Nagarsheth N, Cameron M, et al. An analog of the natural steroidal alkaloid cortistatin A potently suppresses Tat-dependent HIV transcription. Cell Host Microbe. 2012;12:97–108. doi: 10.1016/j.chom.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Campos N, Myburgh R, Garcel A, Vautrin A, Lapasset L, et al. Long lasting control of viral rebound with a new drug ABX464 targeting Rev - mediated viral RNA biogenesis. Retrovirology. 2015;12:30. doi: 10.1186/s12977-015-0159-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lederman MM, Cannon PM, Currier JS, June CH, Kiem HP, et al. A Cure for HIV Infection: “Not in My Lifetime” or “Just Around the Corner”? Pathog Immun. 2016;1:154–64. doi: 10.20411/pai.v1i1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stevenson M. HIV persistence in macrophages. Nature medicine. 2017;23:538–9. doi: 10.1038/nm.4337. [DOI] [PubMed] [Google Scholar]
- 110.Li JZ, Smith DM, Mellors JW. The need for treatment interruption studies and biomarker identification in the search for an HIV cure. AIDS. 2015;29:1429–32. doi: 10.1097/QAD.0000000000000658. [DOI] [PMC free article] [PubMed] [Google Scholar]