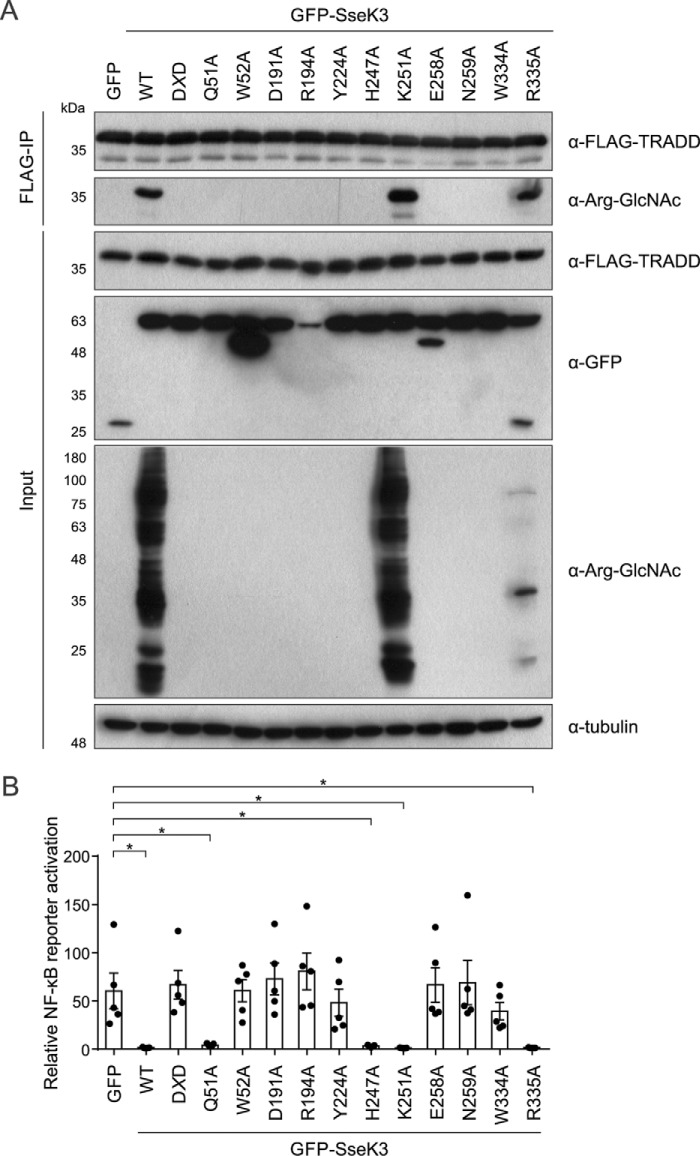
Figure 5.
Screening SseK3 putative catalytic mutants for loss of arginine-GlcNAcylation toward TRADD. A, 293ET cells co-transfected with FLAG-TRADD and the indicated GFP-tagged variants of SseK3 were lysed, and inputs and anti-FLAG immunoprecipitates were analyzed by immunoblotting. Arginine-GlcNAcylation of post-nuclear supernatants (input) and immunoprecipitated FLAG-TRADD was tested using anti-Arg-GlcNAc antibody. Expression of GFP-tagged SseK3 variants was tested using anti-GFP antibody in cell lysates. Antibodies to tubulin were used as a loading control. The shown immunoblots are representative of four independent experiments. B, 293ET cells were co-transfected with an NF-κB–dependent luciferase reporter plasmid, pTK-Renilla luciferase, and the indicated GFP-tagged SseK3 mutants for 24 h before overnight stimulation with 50 ng/ml TNFα. Luciferase activity was measured in cell lysates, and results are presented as fold activation relative to unstimulated controls expressing each SseK3 variant. Data are the mean ± S.E. of five independent experiments, for which individual data points are indicated. Cell lysates from B were analyzed by immunoblotting in Fig. S4. *, p < 0.05 one-way analysis of variance. DXD corresponds to the SseK3 D226A/D228A mutant.