Figure 3.
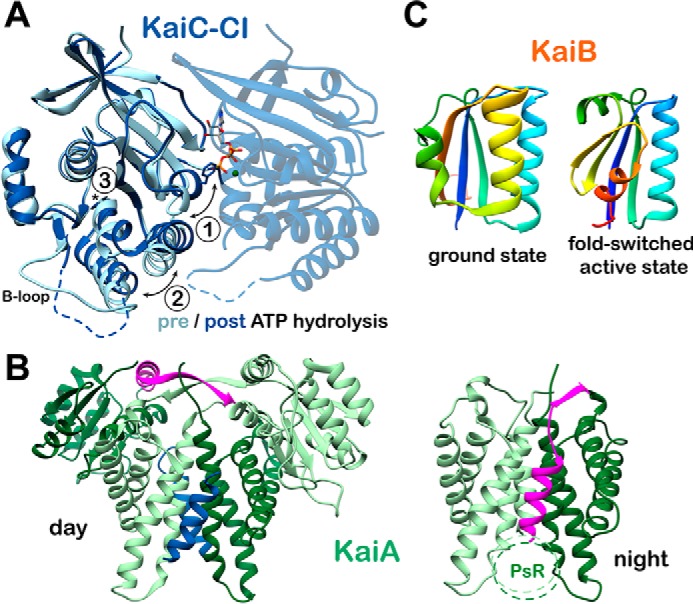
Conformational changes in the core oscillator. A, CI ATP hydrolysis. High-resolution crystal structures of the CI domain of KaiC in the ATP-bound (PDB code 4LTA) and ADP-bound (PDB code 4LT9, chain C) states are overlaid. Three major structural differences are noted upon ATP hydrolysis: 1) helix bearing Phe-199 is flipped into an alternative conformation; 2) α6-α7/α8 helices are repositioned away from the nucleotide-binding site; and 3) cis peptide bond between Asp-145 and Ser-146 in the ATP-bound state is found in the trans isomer in the ADP-bound state. B, KaiA inactivation. The daytime and nighttime states of KaiA are compared. During the day, the A-loops of KaiC (blue) are bound at the dimer interface, and the interdomain linker (pink) crosses over the complex. In the Night Complex, the linker shifts to occupy the A-loop binding site. C, KaiB fold-switching. KaiB undergoes a major structural reorganization between its free and KaiC-bound forms, involving changes in both secondary and tertiary structure for the C-terminal half of the protein. Both structures are represented with rainbow coloring, starting with dark blue at the N terminus. Note that the first half of the two proteins is identical (dark blue through light green), whereas the C-terminal halves are completely different. This interconversion is thought to happen spontaneously resulting in a conformational selection mechanism for formation of the KaiBC complex.
