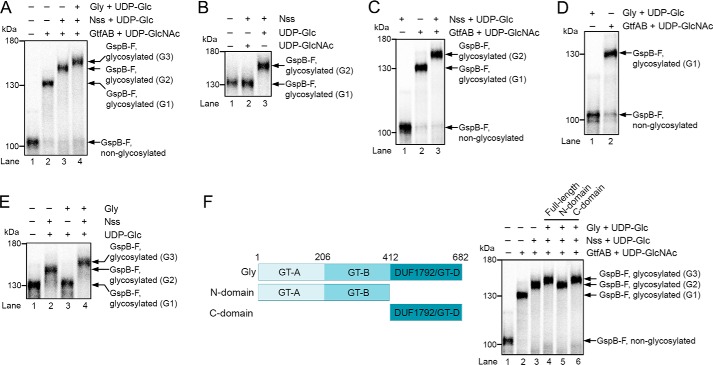
Figure 1.
Adhesin is sequentially O-glycosylated by three glycosyltransferases. A, fragment of the S. gordonii adhesin GspB (GspB-F) was synthesized in reticulocyte lysate in the presence of [35S]methionine. After translation, the glycosyltransferases GtfA/B, Nss, and Gly were added, as indicated, together with UDP-GlcNAc or UDP-Glc. The samples were analyzed by SDS-PAGE and autoradiography. G1, G2, and G3 indicate different glycosylated species. B, in vitro synthesized GspB-F was incubated with Nss and either UDP-Glc or UDP-GlcNAc. C, in vitro synthesized GspB-F was incubated with UDP-sugars and Nss in the absence or presence of GtfA/B. D, in vitro synthesized GspB-F was modified with GtfA/B or Gly in the presence of UDP-GlcNAc or UDP-Glc, respectively. E, in vitro synthesized GspB-F was modified with GtfA/B and further incubated in the presence of UDP-Glc with either Nss or Gly. F, left panel shows the domain organization of Gly. The right panel shows in vitro synthesized GspB-F that was incubated with GtfA/B, Nss, and either full-length Gly or its N- or C-terminal domains, together with UDP-Glc or UDP-GlcNAc.