Figure 2.
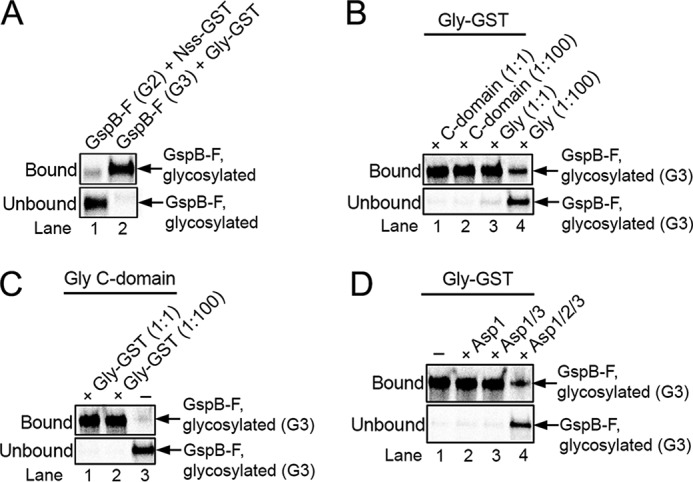
Glycosyltransferase Gly remains associated with its enzymatic product. A, Gsp-F was synthesized in vitro in the presence of [35S]methionine and then glycosylated with GtfA/B to generate the G1 species, followed by incubation with either a GST fusion to Nss (Nss-GST; lane 1) or wildtype Nss (lane 2) to generate the G2 species. The sample in lane 2 was further incubated with a GST fusion to Gly (Gly-GST) to generate the G3 species. The samples were then incubated with glutathione beads, and the bound and unbound fractions were analyzed by SDS-PAGE and autoradiography. The G2 and G3 species migrated relative to molecular weight markers as in Fig. 1A. B, G3 species was generated with GtfA/B, Nss, and GST-Gly and bound to glutathione beads. After washing, the beads were incubated with either the same amount (1:1) or a 100-fold excess (1:100) of His-tagged versions of either full-length Gly (Gly) or C-terminal Gly domain (C domain). The bound and unbound fractions were analyzed as in A. C, G3 species was generated with a His-tagged version of the C domain of Gly (Gly C domain). The sample was then mixed with either the same amount (1:1) or a 100-fold excess (1:100) of Gly-GST and incubated with glutathione beads. Bound and unbound fractions were analyzed as in A. D, G3 species was generated with Gly-GST and bound to glutathione beads. After washing, the beads were incubated with Asp1, Asp1/3 complex, or Asp1/2/3 complex, and the bound and unbound fractions were analyzed as in A.
