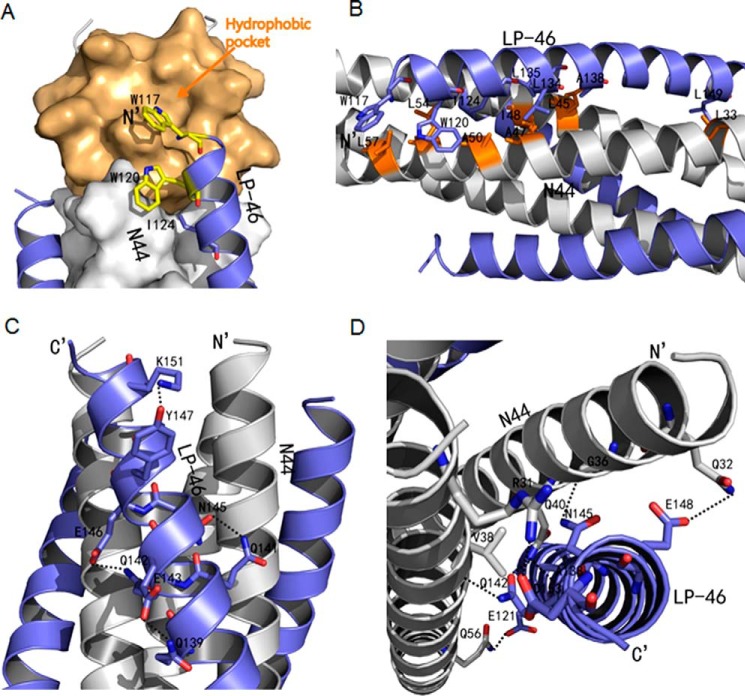
Figure 6.
Interactions between N44 and LP-46. A, binding of the introduced pocket-binding sequence in LP-46 with the NHR pocket site. The N44 trimer is shown as a surface model in gray with the pocket region marked in light orange. LP-46 inhibitors are shown as ribbon models and colored in slate. Residues related to hydrophobic interactions in LP-46 are shown as stick models in yellow. B, hydrophobic contacts of LP-46 with the N44 helices in ribbon models. The N44 peptides are colored in gray with the residues related to hydrophobic interactions marked in orange, whereas the LP-46 inhibitors are in slate. C, intrahelical bonds in the LP-46 helix. The N44 helices are colored in gray, and the LP-46 inhibitors are colored in slate. The residues involving hydrogen bond formation are shown as stick models with labels. Hydrogen bonds are indicated in dashed black lines. D, interhelical bonds between N44 and LP-46. The N44 helices are colored in gray, and the LP-46 inhibitors are colored in slate. The residues involved in interhelical hydrogen bond formation are shown as stick models with labels. Hydrogen bonds are indicated in dashed black lines.