Abstract
The use of rapamycin to extend lifespan and delay age-related disease in mice is well-established despite its potential to impair glucose metabolism which is driven partially due to increased hepatic gluconeogenesis. We tested whether a combination therapeutic approach using rapamycin and metformin could diminish some of the known metabolic defects caused by rapamycin treatment in mice. In genetically heterogeneous HET3 mice, we found that chronic administration of encapsulated rapamycin by diet caused a measurable defect in glucose metabolism in both male and female mice as early as 1 month after treatment. In female mice, this defect was alleviated over time by simultaneous treatment with metformin, also by diet, such that females treated with both drugs where indistinguishable from control mice during glucose tolerance tests. While rapamycin-mediated glucose intolerance was unaffected by metformin in males, we found metformin prevented rapamycin-mediated reduction in insulin and leptin concentrations following 9 months of co-treatment. Recently, the Interventions Testing Program showed that mice treated with metformin and rapamycin live at least as long as those treated with rapamycin alone. Together, our data provide compelling evidence that the pro-longevity effects of rapamycin can be uncoupled from its detrimental effects on metabolism through combined therapeutic approaches.
Keywords: mTOR, gluconeogenesis, AMPK, leptin, adiponectin, interventions, insulin
Introduction
Pharmaceutical inhibition of the mechanistic target of rapamycin (mTOR) is now well established as a valuable tool to better understand ways by which the aging process can be delayed in mammals. In multiple studies, chronic treatment with the mTOR inhibitor rapamycin significantly extends mouse lifespan in both males and females even when begun late in life [1-5]. Moreover, rapamycin delays the progression of several age-related pathologies and may be capable of revitalizing age-related declines in some tissues [5-9]. At the same time, chronic administration of rapamycin is known to have side-effects such as increased incidence of metabolic dysfunction, including glucose intolerance and insulin resistance, likely hindering its use in human populations as an anti-aging treatment. There is emerging evidence that these metabolic defects caused by rapamycin might be alleviated by adjusting treatment protocols, including use of an intermittent treatment schedule or drug-free holidays [10-12]. It is still unclear whether these alternative treatment regimens fully recapitulate the effects of chronic rapamycin on longevity or healthy aging, though there is growing evidence that at least some of these effects are likely to be maintained [13-15],
Another alternative approach may be to utilize a combination of therapeutics in which the side-effects of chronic rapamycin are treated simultaneously with an additional pharmaceutical. In regards to metabolism, co-treatment with rosiglitazone, a PPARγ ligand and insulin sensitizer, has been shown to partially rescue glucose intolerance and insulin resistance in rapamycin-treated rats [16]. While rosiglitazone is an effective insulin sensitizer, evidence suggests that rapamycin-induced glucose intolerance is mediated through increased hepatic gluconeogenesis [17-19]. Metformin is part of the first-line treatment for type 2 diabetes mellitus and reduces hyperglycemia primarily by inhibiting hepatic gluconeogenesis [20,21]. Independent of this outcome, metformin has also been shown to increase peripheral insulin sensitivity [22], increase peripheral glucose uptake [23], increase fatty acid oxidation [24] and decrease absorption of glucose from the digestive system [25]. The mechanism of action for metformin is still controversial and metformin has been proposed to stimulate the AMP-activated protein kinase (AMPK) pathway by inhibiting complex I activity in the mitochondria [26,27], by inhibition of mitochondrial redox shuttle enzymes [28] or through mitochondria independent mechanisms [29]. Interestingly, metformin also inhibits mTORC1 signaling through S6K1 and 4E‐BP1 and results in decreased protein translation [30]. In contrast to rosiglitazone, metformin is relatively safe, has been used extensively in the US for more than 20 years and its long-term effects in humans have been well-studied [31].
As part of the Interventions Testing Program (ITP), the 2011 longevity cohort tested the effect of combined metformin and rapamycin treatment on the lifespan of HET3 mice [32]. In these recently published data, both female and males in this combined treatment group lived 23% longer than control mice suggesting that combining metformin with rapamycin results in a lifespan extension at least as great as (if not greater than) rapamycin treatment alone. As an extension of these findings, we used this experimental paradigm to test what effect chronic treatment with both pharmaceuticals has on metabolic function of mice. We report here that metformin can almost completely abolish glucose intolerance in female HET3 mice treated with rapamycin. In male HET3 mice treated with rapamycin, glucose intolerance is unaffected by metformin treatment; however, metformin does reverse the effect of rapamycin on several metabolic hormones. These data then provide compelling evidence that the pro-longevity effects of rapamycin can be uncoupled from its detrimental effects on metabolism through combined therapeutic approaches.
RESULTS
Four month old male and female HET3 mice were randomly assigned to one of four groups fed the following diets based on LabDiet 5LG6: diet only (Control), 14 ppm encapsulated rapamycin (eRapa), 0.1% metformin (Met), or both 14 ppm encapsulated rapamycin and 0.1% metformin (eRapa+Met). For the duration of the experiment, mice were given access to diet and water ad libitum.
Body weight and composition
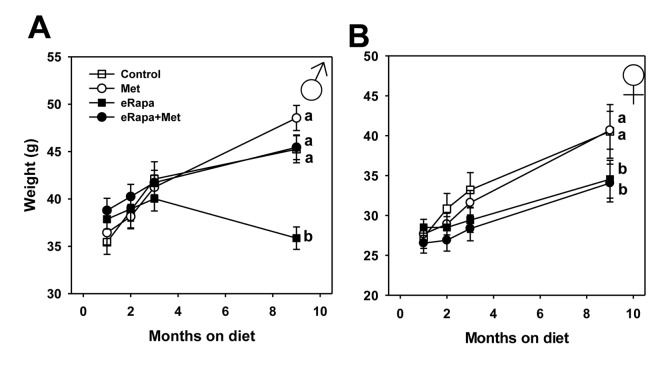
In male mice, the four groups did not differ in weight through 3 months of treatment. After 9 months of treatment, eRapa males weighed significantly less than all other groups including eRapa+Met mice suggesting that metformin abolishes this effect of rapamycin. Females treated with rapamycin, either eRapa or eRapa+Met, weighed significantly less than control and Met-treated females starting at 3 months of treatment and continuing through 9 months (Figure 1). The masses of most fat depots were reduced in mice fed rapamycin at this 9 month point, as were hind-limb skeletal muscle mass in males and kidney mass reduced in female mice fed rapamycin (Supplementary Table 1). Metformin had no effect on tissue mass at this time point except for a small reduction in hind limb muscle mass in females.
Figure 1.
Metformin prevents rapamycin-induced weight loss in male mice. Body weights over time for (A) male and (B) female HET3 mice fed control (open square) diet or diets containing metformin (open circle), rapamycin (closed square), or both metformin and rapamycin (closed circle). Symbols represent mean values at indicated time point ± SEM. For all groups, n=10. Letters indicate significant difference among groups.
Alterations in glucose metabolism
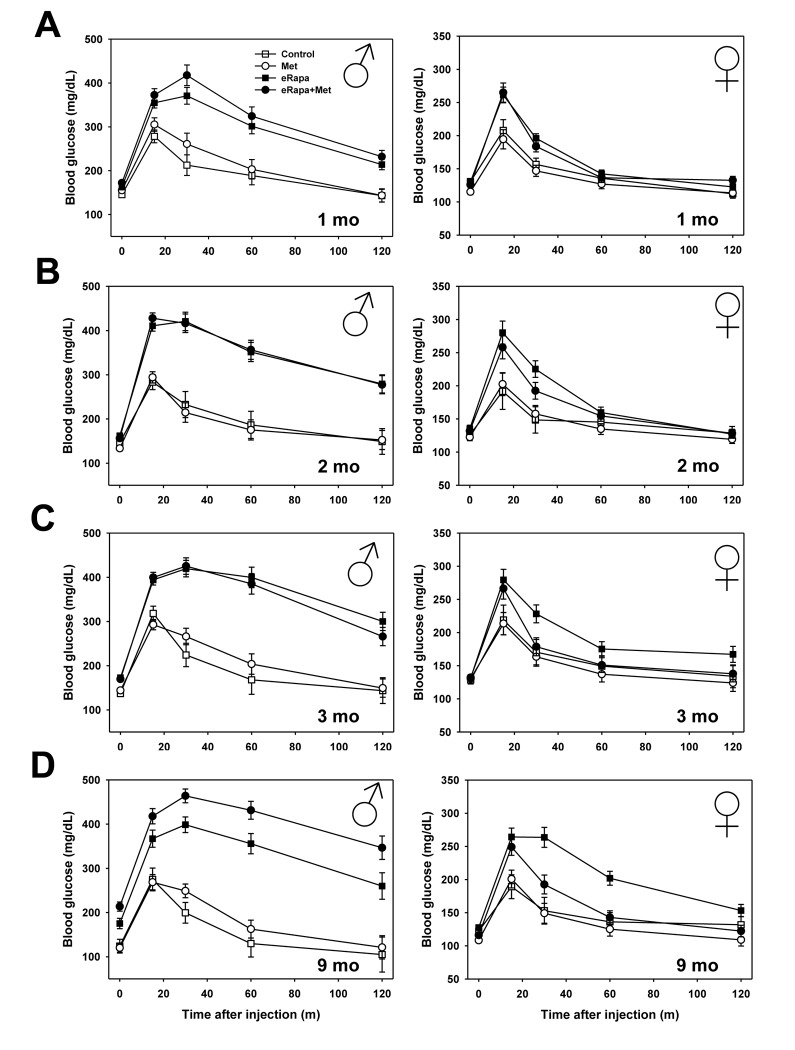
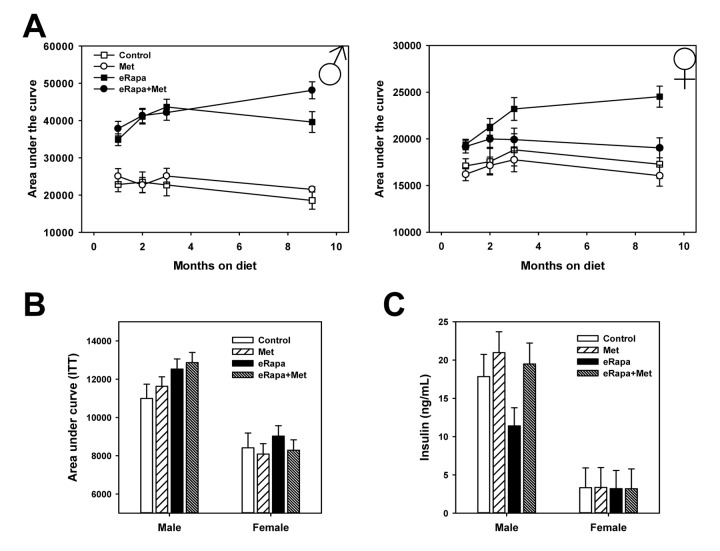
The primary goal of this study was to determine whether metformin could improve the reported defects in glucose metabolism that are caused by short-term and chronic treatment with rapamycin. For both males and females, metformin alone had no effect on glucose tolerance compared to controls at any point during this study. Martin-Montalvo et al. showed a similar outcome in male C57BL/6 mice using the same 0.1% metformin dose [33]. In contrast, both males and females, eRapa treatment caused significant glucose intolerance beginning at our initial assessment point 1 month after treatment. In both sexes, metformin had no effect on glucose tolerance either alone or as an interaction with rapamycin (Figure 2 and Supplementary Table 2). In the same animals, we repeated glucose tolerance tests at 2, 3 and 9 months after treatment was begun (Figure 2 and Figure 3A). In males, rapamycin had a significant effect on the area under curve (AUC) calculated for glucose tolerance tests at all times tested. Metformin had no significant effect either alone or as interaction with rapamycin (Supplementary Table 2).
Figure 2.
Metformin abrogates rapamycin-mediated glucose intolerance in female mice. Glucose tolerance tests performed in male (left) and female (right) mice following (A) 1, (B) 2, (C) 3, or (D) 9 months of indicated diet treatments. Symbols represent mean values for indicated group at each time point ± SEM. For all groups, n=8-10.
Figure 3.
Combined effects of rapamycin and metformin on glucose metabolism. (A) Area under the curve for glucose tolerance tests repeated in the same cohort of animals following indicated months of treatment. Symbols represent mean values for indicated group at each time point ± SEM. (B) Area under the curve calculated for insulin tolerance tests performed following 3 months of treatment. Bars represent mean values for indicated group at each time point ± SEM. (C) Insulin concentration in plasma collected from fed mice following 9 months of treatment on the indicated diets. Bars represent mean values for indicated group at each time point ± SEM. For glucose and insulin tolerance test, n=8-10 for all groups. For insulin measurements, n=8-10 for all groups.
In females, rapamycin had a significant effect on glucose tolerance at all points tested. Metformin had no significant effect through 3 months of treatment, but did have a significant effect at 9 months of treatment. Ad hoc multiple comparison (Holm-Sidak) testing of female treatment groups found a significant effect of metformin among those mice treated with rapamycin (p=0.001) but no effect of metformin within mice not treated with rapamycin (p=0.52). Thus, metformin at this dose reduces the glucose metabolic dysfunction caused by rapamycin in female, but not male, HET3 mice.
In males, rapamycin increased fasting blood glucose concentrations as early as 1 month on the diet and continued throughout the duration of the experiment (Supplementary Table 3). Metformin had little effect on male fasting glucose at the times measured. In contrast, rapamycin had no effect on fasting blood glucose in female mice throughout the study. Further, metformin reduced female blood glucose concentrations both 1 month and 9 months after treatment was begun. Using insulin tolerance tests, we found that rapamycin causes mild insulin resistance in male mice whether given alone or in conjunction with metformin (Two way ANOVA eRAPA F=5.7, p=0.025; Met F=0.7, p=0.41, interaction F=0.1, p=0.81). In females, neither rapamycin nor metformin had any effect on insulin sensitivity as measured by this test (Figure 3B and Supplementary Figure 1).
Endocrine regulation and hepatic gluconeogenesis
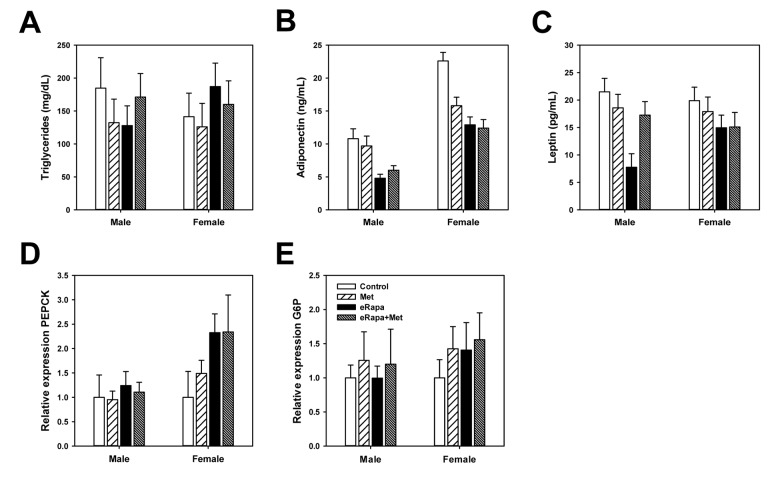
In the interest of identifying the effect of chronic rapamycin and metformin under normal conditions, tissues including plasma were collected from animals in the ad libitum fed rather than fasted conditions. In males, there was no significant effect of rapamycin or metformin on insulin concentrations when analyzed by 2 way ANOVA (Figure 3C). However, when analyzed by ANOVA (i.e., each treatment group as an independent variable) the eRapa treated mice had significantly reduced insulin concentration compared to control (Holm-Sidak multiple comparison p = 0.024). Neither metformin nor rapamycin had any effect on insulin concentrations in female mice. Interestingly, insulin concentrations in females were ~1/5 of that of males corresponding to the relative insulin sensitivity of female HET3 mice compared to males. Plasma triglycerides in both male and female mice were unaffected by treatment (Figure 4A).
Figure 4.
Rapamycin and metformin effects on circulating metabolic markers. (A) Triglycerides, (B) Adiponectin and (C) Leptin in plasma collected from fed mice following 9 months of treatment on the indicated diets. For A-C, n=8-10 for all groups. Rapamycin and metformin effects on hepatic gluconeogenesis. (D) relative phosphoenolpyruvate carboxykinase (PEPCK) expression and (E) relative glucose 6-phosphatase (G6P) expression in liver from male and female mice fed indicated diets. For D and E, n = 5 for all groups. For all, bars represent mean values for indicated group ± SEM.
Adiponectin and leptin are two hormones released by adipose tissue that play key roles in the regulation of glucose metabolism. In males, rapamycin significantly reduced plasma concentrations of adiponectin with no effect of metformin (2 way ANOVA eRAPA F=45.8; p<0.001; metformin F=0.02, p=0.8; interaction F=2.4, p=0.13). Rapamycin also significantly reduced leptin concentrations in males which appeared to be restored by concurrent metformin treatment (2 way ANOVA eRAPA F=13.7, p=0.001; metformin F=2.6, p=0.12; interaction F=9.3, p=0.05) (Figure 4B). In females, both rapamycin and metformin reduced adiponectin concentrations with no significant interaction (2 way ANOVA eRAPA F=16.5, p<0.001; metformin F=5.1, p=0.03; interaction F=3.7, p=0.06). Leptin concentrations in females were unaffected by either metformin or rapamycin (Figure 4C).
Previous studies suggest rapamycin-induced impairments in glucose metabolism are largely due to hepatic insulin resistance and failure of suppression of hepatic gluconeogenesis [17,34]. Gene expression of gluconeogenesis regulators phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6-phosphatase (G6P) were unchanged by treatment in males (Figures 4D and 4E). G6P was unaffected by either treatment in females, but rapamycin increased expression of PEPCK in females with no effect of metformin (2 way ANOVA eRAPA F=4.3, p=0.05; metformin F=0.2, p=0.65; interaction F=0.2, p=0.65).
mTOR and AMPK signaling
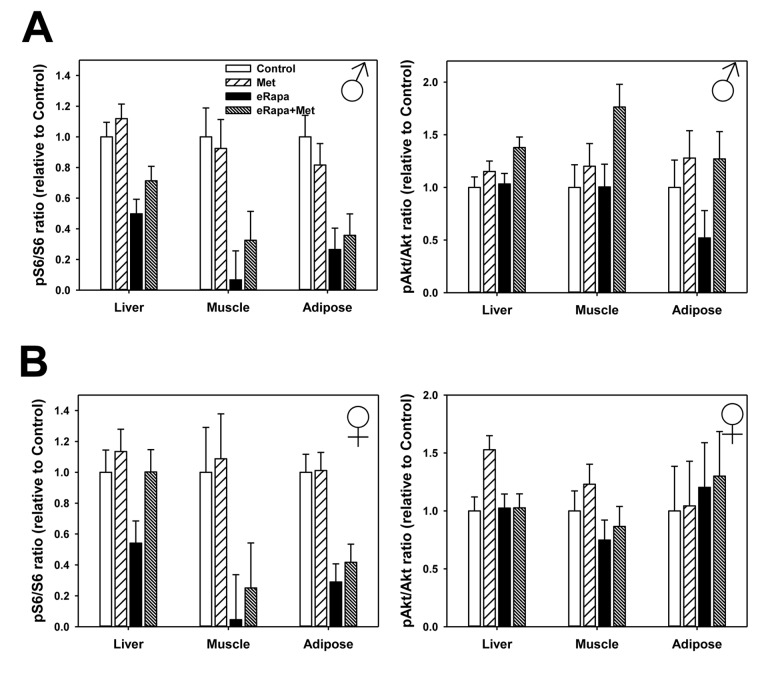
In males, rapamycin generally reduced mTORC1 signaling (as measured by p-S6) in liver, skeletal muscle and adipose tissue (Figure 5, Supplementary Figure 2 and Supplementary Table 4). Metformin had no significant effect nor was there an interaction effect in males suggesting this drug did not alter S6 phosphorylation or the effect of rapamycin. In females, rapamycin significantly reduced mTORC1 in skeletal muscle and adipose and similar to males there was no effect of metformin or interaction in these tissues. In female liver, we found no significant effect of rapamycin on phosphorylation of S6 when analyzed by 2 way ANOVA. However, when only control females and the eRapa females were compared by t-test, there was a significant (p=0.025) reduction in S6 phosphorylation and no significant difference between control and eRapa+Met groups.
Figure 5.
No effect of metformin on rapamycin-mediated mTORC1 inhibition. Quantification of phosphorylation/total protein ratios for S6 (left) and Akt (right) for liver, adipose tissue and muscle collected from male (A) and female (B) mice. Bars represent mean values for indicated diet/sex ± SEM. For all groups, n=6.
We found that rapamycin had much less effect on mTORC2 signaling (as measured by Ser473 phosphorylation of Akt) in both sexes with the only significant effect on a small increase in female adipose. Metformin also had little effect on mTORC2 with the only significant effect a small increase in male liver (Figure 5, Supplementary Figure 2 and Supplementary Table 5).
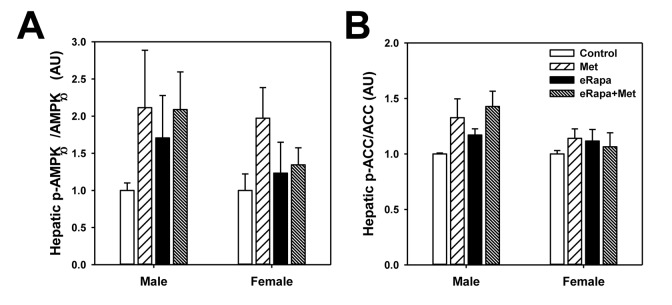
While the mechanism action of metformin is incompletely understood, it has been reasonably well-accepted that metformin activates the AMP-activated protein kinase (AMPK) signaling pathway particularly in the liver. In male mice, phosphorylation of the AMPKα subunit was unchanged by either rapamycin or metformin (2 way ANOVA eRAPA F=0.4, p=0.54, metformin F=1.9, p=0.19, interaction F=0.4, p=0.51) (Figure 6 and Supplementary Figure 2). We found a similar results for AMPKα subunit phosphorylation in females (2 way ANOVA eRAPA F=0.4, p=0.57, metformin F=2.6, p=0.13, interaction F=1.7, p=0.22). However, metformin significantly increased phosphorylation of one of the effectors of AMPK, Acetyl-CoA carboxylase (ACC), in male (2 way ANOVA eRAPA F=1.4, p=0.25, metformin F=6.7, p=0.024, interaction F=0.1, p=0.76), but not female mice. In general, these data point to only moderate activation of AMPK signaling at this dose of metformin delivered through the diet.
Figure 6.
Mild activation of AMPK signaling in metformin treated mice. Quantification of phosphorylation/total protein ratios for (A) AMPKα and (B) ACC from liver of male and female mice fed indicated diets. Bars represent mean values for diet/sex ± SEM. For all groups, n=6.
One potential reason for the differences between males and females in the outcomes we report above might be sex-dependent differences in metabolism of rapamycin [1]. Unlike previous reports using this encapsulated version of rapamycin, we found no difference in blood rapamycin concentration between male and female mice treated with rapamycin alone (Table 1). Surprisingly, the combination of metformin and rapamycin resulted in significantly reduced blood concentrations of rapamycin in females though not males. While this could partially explain the reduced mTOR inhibition in female liver, it is not clear why we did not also not similar decreases in mTOR signaling in female muscle and adipose. Perhaps even more surprisingly, the concentration of metformin was significantly reduced in animals treated with both drugs in comparison to those treated with metformin alone. This occurred in both male and female mice.
Table 1. Blood concentrations of rapamycin and metformin at sacrifice.
| Group | Rapamycin [ng/mL] | p value: eRapa vs eRapa+Met | Metformin [µg/mL] | p value: Met vs eRapa+Met |
| Male | ||||
| eRapa | 38 ± 7 | - | ||
| Met | - | 2.1 ± 0.3 | ||
| eRapa+Met | 41 ± 7 | 0.79 | 1.3 ± 0.2 | 0.04 |
| Female | ||||
| eRapa | 43 ± 8 | - | ||
| Met | - | 3.9 ± 1.0 | ||
| eRapa+Met | 27 ± 4 | 0.07 | 1.3 ± 0.2 | 0.07 |
p value presented is for t test comparing values of indicated groups. Dash indicates levels of this drug in indicated group fall below level of detection. Control groups for both males and females have undetectable levels of both rapamycin and metformin.
DISCUSSION
Our main finding here is that a multi-drug approach utilizing metformin can alleviate common metabolic deficits associated with chronic rapamycin treatment as a pro-longevity therapeutic. Moreover our data are in line with recent studies suggesting the metabolic dysfunction of rapamycin can be dissociated from its molecular effects on inhibition of mTOR either pharmaceutically or through alternative pharmaceutical regimens [10,16]. This has been an ambiguous question since the initial report of the pro-longevity effect of rapamycin administration in mice by the ITP in 2009 [1]. One interpretation of this seemingly paradoxical outcome is that rapamycin promotes long life despite also developing glucose intolerance, insulin resistance and/or decline in insulin production and that preventing these metabolic defects may extend lifespan further still. While glucose impairments promote clear health deficits in humans, one potential confound of this interpretation for mouse studies is that their lifespan is typically thought to be due to the development of cancer rather glucose metabolic dysfunction. However, such metabolic impairments have been associated with the acceleration of cancer progression and even the development of certain types of cancer in mouse models suggesting that glucose metabolism could impact mouse mortality at least indirectly [35,36]. The ITP has recently reported the lifespan of eRapa+Met mice is at least as long, if not longer than historical data for mice treated with eRapa alone [32]. Together, our data provide modest evidence for this interpretation, but leave open questions regarding the next steps in research pursuit. While both young and old mice are susceptible to rapamycin-mediated glucose intolerance [34], it is unknown whether metformin would have the same benefit in aged mice. More testing will be required to address whether metformin can alleviate the greater degree of glucose intolerance caused by higher doses of rapamycin than the 14 ppm dose used here [37].
The effects of rapamycin on glucose intolerance have been largely attributed to the inhibition of mTORC2, rather than mTORC1, that occurs with chronic administration of rapamycin [17]. Genetic ablation of mTORC2 in mice is sufficient to cause glucose intolerance in both sexes and shorten lifespan in male, but not females [18]. Beyond glucose intolerance in our mice, we found only relatively minor effects of rapamycin on markers of gluconeogenesis. One possibility for these small effects may be the relative lack of mTORC2 inhibition we noted in our rapamycin-treated mice. The effect of rapamycin on mTORC2 does seem to be heavily dependent on several factors including tissue type, genetic background, diet, fed or fasted state [11,18,38]. Our data show strong effects of rapamycin on mTORC1 signaling after 9 months in liver, muscle and adipose but relatively little effect of rapamycin (or metformin) on mTORC2. However, the tissues we used were collected from mice in a time restricted fed, rather than fasted, state. Schreiber et al. recently showed that harvesting conditions, particularly whether mice were insulin injected or not, can dramatically alter the outcome of rapamycin-mediated mTORC2 inhibition in vivo [38]. Moreover, a careful examination of potential compensatory effects on mTORC2 signaling due to such long-term (9 months) treatment with chronic rapamycin have not yet been performed.
Interestingly, we show several disparities between male and female mice in terms of both rapamycin as well as combined rapamycin and metformin treatment. It has been well-established that rapamycin extends lifespan to a greater degree in female compared to male mice [1,2,4,37]. It is not clear here why only females benefited from metformin treatment in regards to rapamycin-mediated glucose intolerance, however it seems likely to be related to reduced severity of glucose dysfunction in female mice treated with rapamycin compared to males [37,39]. Here, rapamycin did not increase fasting blood glucose in female mice at any time and glucose intolerance in female mice was approximately two-fold less than that caused in male mice. Interestingly, metformin did not immediately alleviate the effects of rapamycin on glucose intolerance. As Figure 3A shows, rapamycin-treated mice became progressively more glucose intolerant over the course of 9 months; metformin showed no effect early but cumulative use of metformin seemed to blocks this progression. Short-term treatment with metformin is known to have some beneficial effects, though the long-term benefits, including potentially lifespan extension, are associated with cumulative use [40,41]. Our study was limited in sample availability, so an important future study should address the molecular and physiological events at each of the time points (or more) used in our study. In previous mouse studies, it has been reported that metformin can preferentially reduce cancer and extend longevity in female but not male mice [42]. However, it is of interest that we also noted male-specific effects of combined metformin and rapamycin treatment. Rapamycin-mediated changes in body weight and insulin concentration all seemed to be altered by metformin in males but not females. The changes in leptin associated with the combined treatment in male mice is likely related to the difference in body weight, though further study would be required to delineate this relationship. It is unclear on why metformin also seems to counter the loss of weight caused by rapamycin in male mice. While we did not collect food consumption data, it is possible that cell non-autonomous mechanisms, in the brain for example, that regulate food intake could be affected by both drugs. Again, it is unclear why this would preferentially affect male and not female mice in this study. It would be of interest to test whether longer treatments or higher doses of metformin might also alleviate glucose intolerance in male mice. However, it must be noted that a metformin dose 10-fold higher than used here has been reported as toxic to mice [33].
Perhaps most interesting in this study is the finding that combined treatment with metformin and rapamycin reduces the effective concentration of rapamycin (in females) and metformin (in both sexes) relative to treatment with either drug alone. While mathematically the diet which contains both drugs would have less of each drug available on a per gram basis, this difference is relatively tiny and unlikely to produce such a large difference. More likely, there is likely drug interaction between metformin and rapamycin that in some way impairs absorption or metabolism of these compounds. While these drugs have not been reported to interact directly, there are several drug interactions known to alter metabolism of metformin [43]. Importantly, the final concentrations of metformin and rapamycin even in combination are within the range of effective concentrations clinically. In diabetic patients prescribed metformin as part of their treatment, clinically relevant plasma concentrations of metformin are reported to be in the range of 10 µM, or approximately 1.3 mg/mL [44].
In contrast to previous studies, we found no evidence that metformin inhibited mTOR signaling when given as a single therapeutic [30]. Interestingly, we did find that metformin appeared to impair the ability of rapamycin to inhibit mTOR in the liver when both drugs were given concurrently. It is not clear why only this tissue, and this sex, seemed to show this effect. However, this could partially explain why female, but not male, mice benefitted metabolically from metformin. It would be of interest to further test whether metformin could alleviate the gluconeogenesis defect previously shown in some mTOR mutant mice [18]. This lack of effect on mTOR signaling may also partially explain why metformin at this dose failed to extend longevity in a similar cohort of HET3 mice [32]. One interpretation might be that metformin has no, or only mild, effects on physiological markers such as metabolism or longevity unless under a mildly stressful conditions, i.e., diabetes or metabolic dysfunction caused by rapamycin. Under this scenario, metformin may be more beneficial to aging and longevity in the human condition where there are no strict dietary and environmental controls as found in the animal vivarium. Certainly upcoming human studies could be used to address this type of question [45]. However, our study here shows that metformin is certainly valuable as part of a combined treatment with rapamycin and overall contributes to growing sets of data that alternative treatment regimens with rapamycin may be used to maximize effects on longevity and minimize side-effects [10,13,14,32,46].
METHODS
Mice and diets
Details of breeding and husbandry have been described elsewhere [1,2,37]. Males and females were separated at weaning and housed at a cage density of five mice per cage. All experiments were approved by the Institutional Animal Care and Use Committees at UTHSCSA and were performed under the supervision of the animal core facility of the Barshop Institute at UTHSCSA. At four months of age, mice were fed either LabDiet 5LG6 (PMI Nutrition International, Bentwood, MO, USA) diet alone or this diet containing either rapamycin (14 ppm), metformin (0.1% or 1000 ppm) or a combination of both rapamycin and metformin at these doses. Further discussion of diet formulations are discussed in [32].
Assessment of glucose metabolism
Glucose and insulin tolerance tests were performed by fasting the mice for 6 hours (8:00-14:00) and then injecting either glucose (1.5 g kg-1) or insulin (0.75U kg-1) intraperitoneally as previously described [47]. Glucose measurements were performed at the specific time points indicated using a handheld OneTouch Ultra glucometer (LifeScan, Inc., Milpitas, CA) and test strips. Glucose and insulin tolerance tests were repeated in mice at 1, 2, 3 and 9 months after treatment was initiated. Areas under curve were calculated using the trapezoidal method from absolute glucose data determining during each tolerance test. Mice were euthanized after 9 months of treatment and tissues collected, processed and frozen at -80° C for future use. All mice were euthanized in the morning (09:00-11:00) and were given ad libitum access to food and water prior to sacrifice. Insulin (Crystal Chem, Downer’s Grove IL), triglycerides (Sigma, St. Louis MO), adiponectin and leptin (Millipore, Billerica MA) kits were used to measure metabolite concentrations in plasma per manufacturer’s instructions. Hepatic pyruvate and lactate were measured using commercial assay kits (Cayman Chemicals, Ann Arbor MI).
Immunoblot
Total protein extracts from skeletal muscle, adipose and liver were created by homogenization of frozen tissue samples in RIPA buffer with additional protease and phosphatase inhibitors (Thermo Scientific, Rockford IL), followed by centrifuged at 14,000g at 4°C for 15 minutes. Protein content of the resultant supernatant was measured by BCA assay and equal amounts of protein samples were separated by SDS-PAGE, transferred to polyvinylidene difluoride membrane (Millipore) and subjected to immunoblot using the following antibodies and catalog numbers (Cell Signaling, Beverely MA): phosphorylated (Ser 235/236; #CS4857) and total ribosomal protein S6 (#CS2217), phosphorylated (Ser473; #CS9731) and total Akt (#CS9272), phosphorylated AMPKα (Thr172; #CS2535) and total AMPKα (#CS5831), and phosphorylated Acetyl-CoA carboxylase (Ser79; #CS11818) and total ACC (#CS3676). Protein bands were visualized using alkaline phosphatase-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz CA) and ECL reagent. All immunoblots were quantified using ImageJ (NIH).
RT-PCR
Total RNA was prepared from liver using TriReagent (Sigma) which was then treated with DNase I (Invitrogen, Carlsbad CA). cDNA was generated using Retroscript for RT-PCR (ThermoFisher, Waltham MA). Primers for PEPCK: (F) 5’-3’:TCTCTGATCCAGACCTTCCAA; (R) 5’-3’: GAAGTCCAGACCGTTATGCAG. Primers for G6P: (F) 5’-3’: GAAGGCCAAGAGATGGTGTGA; (R) 5’-3’:TGCAGCTCTTGCGGTACATG. Expression of actin as a housekeeping gene was used for normalization. Real-time PCR was performed in Applied Biosystems 7900 Real-Time PCR system with default PCR program. Triplicate samples were run in in 384-well real time PCR plate using a final volume of 10 µl with 16.26 µl SYBR Green PCR Master Mix (Applied Biosystems, Waltham MA), 160 nM forward primer, 160 nM reverse primer, and 8 ng template cDNA in each well. Ct values generated from these PCR were analyzed by relative quantification to values generated for control mice of each sex.
Rapamycin blood concentrations
Whole blood was collected at sacrifice and analyzed for rapamycin and metformin concentrations by the San Antonio Nathan Shock Center Bioanalytical Pharmacology Core [1,48].
Statistical analysis
Male and female data were analyzed separately by 2 way ANOVA using rapamycin and metformin treatment as the two subject factors. Male and female data were analyzed separately due to well-known sex difference in both the basic physiology of this strain of mice (HET3) as well as the difference in effect of rapamycin published elsewhere. Post-hoc analyses were performed using the method of Holm-Sidak. Where applicable, Student t-tests were used to compare 2 groups of interest. Statistical significance was attributed to all data for which a p value of less than or equal to 0.05.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Vivian Diaz and the staff of the San Antonio Nathan Shock Center Animal Core (P30 AG013319) for assistance in animal husbandry. The authors also thank the Interventions Testing Program (U01 AG022307) for developing the diets used in this experiment and sharing of resources.
Footnotes
AUTHOR CONTRIBUTIONS: RW, EF, YL, and ABS performed experiments. EF, RS, and ABS contributed to experimental design. RW and ABS analyzed data. RW, RS and ABS prepared and reviewed manuscript.
CONFLICTS OF INTEREST: The University of Texas Health Science Center at San Antonio has applied for a patent, U.S. Patent Application No. 13/128,800, with co-inventor RS, for an encapsulated rapamycin formulation used in this paper. Under a licensing agreement between Rapamycin Holdings, Inc. and the University of Texas Health Science Center San Antonio and RS, the University is entitled to milestone payments and royalty on sales of microencapsulated rapamycin. The university has a plan for managing conflict of interests under its ‘Policy and Procedures for Promoting Objectivity in Research by Managing, Reducing or Eliminating Conflicts of Interest’.
FUNDING: During the course of these studies, ABS was supported by a grant from the National Institute of Health (R01 AG050797), a grant from the American Federation of Aging Research, a grant from the American Heart Association (15BGIA23220016) and the Geriatric Research, Education and Clinical Center of the South Texas Veterans Health Care System. RW was supported by the Medical Student Training in Aging Research (MSTAR) program funded by NIA/AFAR. This material is the result of work supported with resources and the use of facilities at South Texas Veterans Health Care System, San Antonio, Texas. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
REFERENCES
- 1.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009; 460:392–95. 10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011; 66:191–201. 10.1093/gerona/glq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schröder S, Adler T, Afonso LC, Aguilar-Pimentel JA, Becker L, Garrett L, Hans W, Hettich MM, Holtmeier R, et al. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013; 123:3272–91. 10.1172/JCI67674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fok WC, Chen Y, Bokov A, Zhang Y, Salmon AB, Diaz V, Javors M, Wood WH 3rd, Zhang Y, Becker KG, Pérez VI, Richardson A. Mice fed rapamycin have an increase in lifespan associated with major changes in the liver transcriptome. PLoS One. 2014; 9:e83988. 10.1371/journal.pone.0083988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, Diaz V, Sloane L, Maslin K, Treaster S, Réndon S, van Remmen H, Ward W, et al. Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 2014; 69:119–30. 10.1093/gerona/glt056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer KE, Gelfond JA, Soto VY, Han C, Someya S, Richardson A, Austad SN. Health Effects of Long-Term Rapamycin Treatment: The Impact on Mouse Health of Enteric Rapamycin Treatment from Four Months of Age throughout Life. PLoS One. 2015; 10:e0126644. 10.1371/journal.pone.0126644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majumder S, Caccamo A, Medina DX, Benavides AD, Javors MA, Kraig E, Strong R, Richardson A, Oddo S. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1β and enhancing NMDA signaling. Aging Cell. 2012; 11:326–35. 10.1111/j.1474-9726.2011.00791.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai DF, Karunadharma PP, Chiao YA, Basisty N, Crispin D, Hsieh EJ, Chen T, Gu H, Djukovic D, Raftery D, Beyer RP, MacCoss MJ, Rabinovitch PS. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell. 2014; 13:529–39. 10.1111/acel.12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn JM, O’Leary MN, Zambataro CA, Academia EC, Presley MP, Garrett BJ, Zykovich A, Mooney SD, Strong R, Rosen CJ, Kapahi P, Nelson MD, Kennedy BK, Melov S. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell. 2013; 12:851–62. 10.1111/acel.12109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arriola Apelo SI, Neuman JC, Baar EL, Syed FA, Cummings NE, Brar HK, Pumper CP, Kimple ME, Lamming DW. Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system. Aging Cell. 2016; 15:28–38. 10.1111/acel.12405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Diaz V, Fernandez E, Strong R, Ye L, Baur JA, Lamming DW, Richardson A, Salmon AB. Rapamycin-induced metabolic defects are reversible in both lean and obese mice. Aging (Albany NY). 2014; 6:742–54. 10.18632/aging.100688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang SB, Lee HY, Young DM, Tien AC, Rowson-Baldwin A, Shu YY, Jan YN, Jan LY. Rapamycin induces glucose intolerance in mice by reducing islet mass, insulin content, and insulin sensitivity. J Mol Med (Berl). 2012; 90:575–85. 10.1007/s00109-011-0834-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leontieva OV, Paszkiewicz GM, Blagosklonny MV. Weekly administration of rapamycin improves survival and biomarkers in obese male mice on high-fat diet. Aging Cell. 2014; 13:616–22. 10.1111/acel.12211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arriola Apelo SI, Pumper CP, Baar EL, Cummings NE, Lamming DW. Intermittent Administration of Rapamycin Extends the Life Span of Female C57BL/6J Mice. J Gerontol A Biol Sci Med Sci. 2016; 71:876–81. 10.1093/gerona/glw064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiao YA, Kolwicz SC, Basisty N, Gagnidze A, Zhang J, Gu H, Djukovic D, Beyer RP, Raftery D, MacCoss M, Tian R, Rabinovitch PS. Rapamycin transiently induces mitochondrial remodeling to reprogram energy metabolism in old hearts. Aging (Albany NY). 2016; 8:314–27. 10.18632/aging.100881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Festuccia WT, Blanchard PG, Belchior T, Chimin P, Paschoal VA, Magdalon J, Hirabara SM, Simões D, St-Pierre P, Carpinelli A, Marette A, Deshaies Y. PPARγ activation attenuates glucose intolerance induced by mTOR inhibition with rapamycin in rats. Am J Physiol Endocrinol Metab. 2014; 306:E1046–54. 10.1152/ajpendo.00683.2013 [DOI] [PubMed] [Google Scholar]
- 17.Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, Guertin DA, Sabatini DM, Baur JA. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012; 335:1638–43. 10.1126/science.1215135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamming DW, Mihaylova MM, Katajisto P, Baar EL, Yilmaz OH, Hutchins A, Gultekin Y, Gaither R, Sabatini DM. Depletion of Rictor, an essential protein component of mTORC2, decreases male lifespan. Aging Cell. 2014; 13:911–17. 10.1111/acel.12256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houde VP, Brûlé S, Festuccia WT, Blanchard PG, Bellmann K, Deshaies Y, Marette A. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes. 2010; 59:1338–48. 10.2337/db09-1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cusi K, DeFronzo RA. Metformin: a review of its metabolic effects. Diabetes Rev (Alex). 1998; 6:89–131. [Google Scholar]
- 21.Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR, Shulman GI. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000; 49:2063–69. 10.2337/diabetes.49.12.2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrmann DA, Cavaghan MK, Imperial J, Sturis J, Rosenfield RL, Polonsky KS. Effects of metformin on insulin secretion, insulin action, and ovarian steroidogenesis in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997; 82:524–30. [DOI] [PubMed] [Google Scholar]
- 23.Hundal HS, Ramlal T, Reyes R, Leiter LA, Klip A. Cellular mechanism of metformin action involves glucose transporter translocation from an intracellular pool to the plasma membrane in L6 muscle cells. Endocrinology. 1992; 131:1165–73. 10.1210/endo.131.3.1505458 [DOI] [PubMed] [Google Scholar]
- 24.Fulgencio JP, Kohl C, Girard J, Pégorier JP. Effect of metformin on fatty acid and glucose metabolism in freshly isolated hepatocytes and on specific gene expression in cultured hepatocytes. Biochem Pharmacol. 2001; 62:439–46. 10.1016/S0006-2952(01)00679-7 [DOI] [PubMed] [Google Scholar]
- 25.Ikeda T, Iwata K, Murakami H. Inhibitory effect of metformin on intestinal glucose absorption in the perfused rat intestine. Biochem Pharmacol. 2000; 59:887–90. 10.1016/S0006-2952(99)00396-2 [DOI] [PubMed] [Google Scholar]
- 26.Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002; 51:2074–81. 10.2337/diabetes.51.7.2074 [DOI] [PubMed] [Google Scholar]
- 27.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000; 348:607–14. 10.1042/bj3480607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, Jurczak MJ, Camporez JP, Lee HY, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014; 510:542–46. 10.1038/nature13270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng S, Cao J, He Q, Xiong L, Chang E, Radovick S, Wondisford FE, He L. Metformin activates AMP-activated protein kinase by promoting formation of the αβγ heterotrimeric complex. J Biol Chem. 2015; 290:3793–802. 10.1074/jbc.M114.604421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, Marette A, Kozma SC, Thomas G. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010; 11:390–401. 10.1016/j.cmet.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inzucchi S, Bergenstal R, Buse J, Diamant M, Ferrannini E, Nauck M, Peters A, Tsapas A, Wender R, Matthews D. Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012; 35:1364–79. 10.2337/dc12-0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, Fernandez E, Flurkey K, Hamilton KL, Lamming DW, Javors MA, de Magalhães JP, Martinez PA, et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016; 15:872–84. 10.1111/acel.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013; 4:2192. 10.1038/ncomms3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamming DW, Ye L, Astle CM, Baur JA, Sabatini DM, Harrison DE. Young and old genetically heterogeneous HET3 mice on a rapamycin diet are glucose intolerant but insulin sensitive. Aging Cell. 2013; 12:712–18. 10.1111/acel.12097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao S, Li A, Liu F, Chen F, Williams M, Zhang C, Kelley Z, Wu CL, Luo R, Xiao H. NCOA5 haploinsufficiency results in glucose intolerance and subsequent hepatocellular carcinoma. Cancer Cell. 2013; 24:725–37. 10.1016/j.ccr.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryu TY, Park J, Scherer PE. Hyperglycemia as a risk factor for cancer progression. Diabetes Metab J. 2014; 38:330–36. 10.4093/dmj.2014.38.5.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, Pletcher S, Salmon AB, Sharp ZD, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014; 13:468–77. 10.1111/acel.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schreiber KH, Ortiz D, Academia EC, Anies AC, Liao CY, Kennedy BK. Rapamycin-mediated mTORC2 inhibition is determined by the relative expression of FK506-binding proteins. Aging Cell. 2015; 14:265–73. 10.1111/acel.12313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schindler CE, Partap U, Patchen BK, Swoap SJ. Chronic rapamycin treatment causes diabetes in male mice. Am J Physiol Regul Integr Comp Physiol. 2014; 307:R434–43. 10.1152/ajpregu.00123.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Claesen M, Gillard P, De Smet F, Callens M, De Moor B, Mathieu C. Mortality in Individuals Treated With Glucose-Lowering Agents: A Large, Controlled Cohort Study. J Clin Endocrinol Metab. 2016; 101:461–69. 10.1210/jc.2015-3184 [DOI] [PubMed] [Google Scholar]
- 41.Bannister CA, Holden SE, Jenkins-Jones S, Morgan CL, Halcox JP, Schernthaner G, Mukherjee J, Currie CJ. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab. 2014; 16:1165–73. 10.1111/dom.12354 [DOI] [PubMed] [Google Scholar]
- 42.Anisimov VN, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Egormin PA, Yurova MV, Rosenfeld SV, Semenchenko AV, Kovalenko IG, Poroshina TE, Berstein LM. Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice. Aging (Albany NY). 2010; 2:945–58. 10.18632/aging.100245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.May M, Schindler C. Clinically and pharmacologically relevant interactions of antidiabetic drugs. Ther Adv Endocrinol Metab. 2016; 7:69–83. 10.1177/2042018816638050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, Furlong TJ, Greenfield JR, Greenup LC, Kirkpatrick CM, Ray JE, Timmins P, Williams KM. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011; 50:81–98. 10.2165/11534750-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 45.Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a Tool to Target Aging. Cell Metab. 2016; 23:1060–65. 10.1016/j.cmet.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson SC, Yanos ME, Kayser EB, Quintana A, Sangesland M, Castanza A, Uhde L, Hui J, Wall VZ, Gagnidze A, Oh K, Wasko BM, Ramos FJ, et al. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science. 2013; 342:1524–28. 10.1126/science.1244360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salmon AB, Lerner C, Ikeno Y, Motch Perrine SM, McCarter R, Sell C. Altered metabolism and resistance to obesity in long-lived mice producing reduced levels of IGF-I. Am J Physiol Endocrinol Metab. 2015; 308:E545–53. 10.1152/ajpendo.00558.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tardif S, Ross C, Bergman P, Fernandez E, Javors M, Salmon A, Spross J, Strong R, Richardson A. Testing efficacy of administration of the antiaging drug rapamycin in a nonhuman primate, the common marmoset. J Gerontol A Biol Sci Med Sci. 2015; 70:577–87. 10.1093/gerona/glu101 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.