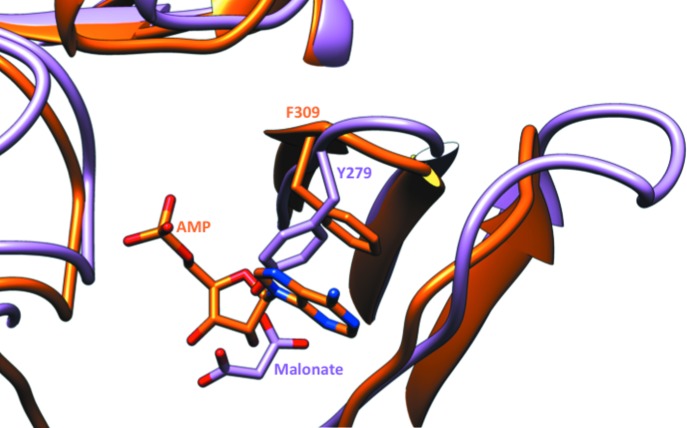
Figure 5.
Allosteric pocket of FPBaseII. Synechocystis FBP/SBPase (orange) has an allosteric site with AMP bound. T84S MtFBPaseII (light purple) in complex with F6P has malonate bound in the same pocket, but the amino-acid residues are not exclusively conserved. Critically, Tyr279 of MtFBPaseII blocks the AMP-binding site present in Synechocystis FBP/SBPase (PDB entry 3rpl), which contains a phenylalanine residue (Phe309) that interacts with the adenine heterocycle by ring stacking.