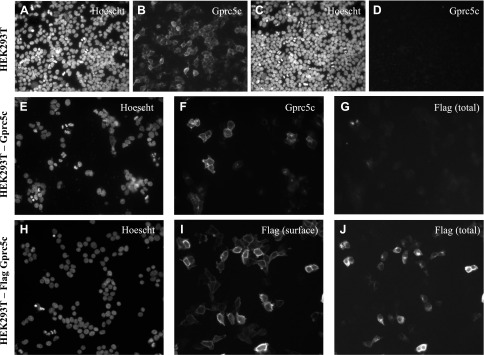
Figure 1.
Confirming the specificity of the Gprc5c antibody and showing that Gprc5c contains a 22-aa long cleavable signal peptide in its N terminus in HEK293T cells. Either the Gprc5c construct (A, B) or the empty vector construct (C, D) was transiently transfected in HEK293T cells, which were then fixed and permeabilized. Cells were stained for nuclei with Hoechst, and Gprc5c expression with the anti-Gprc5c antibody (1:2500). E–G) HEK293T cells were transiently transfected with a Gprc5c construct containing a flag tag on its extreme N terminus and then fixed, permeabilized, and immunostained for both Gprc5c and flag. These cells stained for Gprc5c (F) but not for flag (G); and Hoechst is shown (E). H–J) HEK293T cells were transiently transfected with a Gprc5c construct containing a flag tag between A22 and Q23. Staining for (poly) flag in live, nonpermeabilized cells shows that the flag tag is now present on the cell surface (I). Cells were also stained with a different (mono) flag antibody following fixative and permeabilization, revealing addition flag staining intracellularly (J); and nuclear labeling is shown (H). These data confirm that Gprc5c contains a 22-aa-long cleavable signal peptide on its extreme N terminus. Original magnification, ×20.