Figure 8.
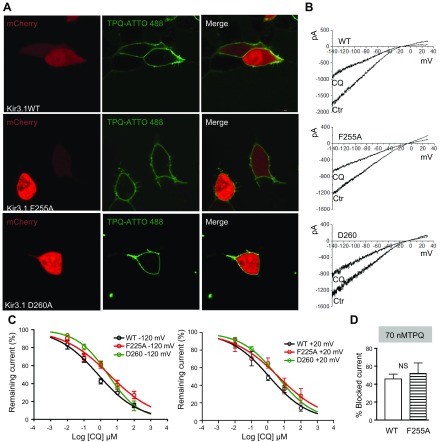
F255A and D260A mutations reduce the ability of chloroquine to block IKACh. A) Confocal microscopy. Fluorescence staining of IKACh proteins. Top) Live HEK293 cells transfected with mCherry, WT Kir3.1, and Kir3.4. Middle (bottom): cells transfected with mCherry, Kir3.1 F255A or D260A, and Kir3.4, where tertiapinQ ATTO-488 showed robust staining of the cell membranes. B) BaCl2-sensitive IKACh currents elicited in WT Kir.31/Kir3.4-, F255A Kir3.1/Kir3.4-, or D260A Kir3.1/Kir3.4-transfected cells, in response ramps from −140 to +30 mV, before and after addition of 1 μM CQ. C, left: Dose-response curves for the effect of chloroquine on the BaCl2-sensitive inward current measured at −120 mV. IC50 for WT: 0.8 μM, R2 = 0.81, n = 11; F255A: 3.2 μM, R2 = 0.75, n = 10; and D260: 2.8 μM, R2 = 0.9, n = 10. P < 0.01 for WT vs. F255A and D260. Right: dose-response curves for outward current measured at +20 mV. IC50 for WT: 1.1 μM, R2 = 0.83, n = 7; F255A: 4.2 μM, R2 = 0.65, n = 7; and D260: 3.8 μM, R2 = 0.89, n = 7. P < 0.01 for WT vs. F255A and D260. D) TertiapinQ (70 nM) block of WT (46.2 ± 5.1%, n = 5) and F255A (52.3 ± 11.3%, n = 5; P = 0.6) Kir3.1/ Kir3.4 currents at −120 mV.