Summary
We have previously shown that a homolog of a conserved Nucleoside-diphosphate-kinase (Ndk) family of multifunctional enzymes and secreted molecule in Porphyromonas gingivalis can modulate selective host molecular pathways including downregulation of reactive-oxygen-species generation to promote bacterial survival in human gingival-epithelial-cells (GECs). In this study, we describe a novel kinase function for bacterial effector, P. gingivalis-Ndk, in abrogating epithelial cell death by phosphorylating Heat-shock-protein-27 (HSP27) in GECs. Infection by P. gingivalis was recently suggested to increase phosphorylation of HSP27 in cancer-epithelial cells; however, the mechanism and biological significance of anti-apoptotic phospho-HSP27 during infection has never been characterized. Interestingly, using GST-rNdk Pull-down analyzed by mass-spectrometry, we identified HSP27 in GECs as a strong binder of P. gingivalis-Ndk and further verified using confocal microscopy and ELISA. Therefore, we hypothesized P. gingivalis-Ndk can phosphorylate HSP27 for inhibition of apoptosis in GECs. We further employed P. gingivalis-Ndk protein constructs and an isogenic P. gingivalis-ndk-deficient-mutant strain for functional examination. P. gingivalis-infected GECs displayed significantly increased phospho-HSP27 compared to ndk-deficient-strain during 24 hours infection. Phospho-HSP27 was significantly increased by transfection of GFP-tagged-Ndk into uninfected-GECs, and in vitro phosphorylation assays revealed direct phosphorylation of HSP27 at serines 78 and 82 by P. gingivalis-Ndk. Depletion of HSP27 via siRNA significantly reversed resistance against staurosporine-mediated-apoptosis during infection. Transfection of recombinant P. gingivalis-Ndk protein into GECs substantially decreased staurosporine-induced-apoptosis. Finally, ndk-deficient-mutant strain was unable to inhibit staurosporine-induced Cytochrome C release/Caspase-9 activation. Thus, we show for the first time, the phosphorylation of HSP27 by a bacterial effector -P. gingivalis-Ndk- and a novel function of Ndks that is directly involved in inhibition of host cell apoptosis and the subsequent bacterial survival.
Introduction
Nucleoside diphosphate kinases are highly conserved multifunctional enzymes expressed in both eukaryotes and prokaryotes which balance the pools of nucleoside triphosphates (NTPs), such as ATP or GTP, by catalyzing the transfer of orthophosphates (Chakrabarty, AM, 1998; Ray, NB et al., 1992; Spooner, R et al., 2012). Ndks form either homo-tetramers or -hexamers with phosphotransferase activity that function in a variety of cellular housekeeping functions such as DNA cleavage/repair, transcriptional regulation, cell proliferation, and apoptosis (Atanasova, K et al., 2016; Kumar, P et al., 2005; Yu, H et al., 2017). In addition to homeostatic functions, bacterial and human Ndk homologs have been implicated in the progression of a variety of different chronic diseases (e.g. cancer) and opportunistic infections (Atanasova, KR et al., 2014; Chakrabarty, AM, 1998; Yu, H et al., 2017). Bacterial Ndks are cytoplasmic proteins of bacteria that are also secreted, as is the case with pathogens such as Mycobacterium tuberculosis, Pseudomonas aeruginosa, and Porphyromonas gingivalis involved in chronic opportunistic infections (Spooner, R et al., 2012; Sun, J et al., 2013). In the context of host-pathogen interaction specifically, these prokaryotic Ndk homologs play roles in immune evasion, inhibition of reactive-oxygen-species (ROS) through NADPH-oxidase complexes, and regulation of host apoptotic factors (Chakrabarty, AM, 1998; Choi, CH et al., 2013; Keith, KE et al., 2009; Sun, J et al., 2013; Yilmaz, O et al., 2008; Yu, H et al., 2017). Typically, bacterial Ndks regulate bacterial proteins through phosphorylation on histidine residues by transferring phosphates from active sites (ATP or GTP) on the enzyme to the histidine residue (Attwood, PV et al., 2015). In eukaryotes, however, serine, threonine, and tyrosine residues are considered the major targets for phosphorylation, while histidine phosphorylation is less defined (Adam, K et al., 2017). Human Ndk (encoded by the nm23 gene) has previously been shown to extend outside of the standard histidine kinase function and phosphorylate on serine/threonine residues as well (Engel, M et al., 1995). Intriguingly, bacterial Ndks have not been shown to phosphorylate on any residues other than histidine; however the significantly conserved homology of eukaryotic and prokaryotic Ndks could suggest shared kinase functions (Schneider, B et al., 2001). To date, this has never been investigated.
The cellular biology of Gram (−) opportunistic bacterium, P. gingivalis in the oral mucosal cells is complex. It has been shown that intracellular invasion of P. gingivalis does not induce apoptosis or necrosis and can render human primary gingival epithelial cells (GECs) resistant to cell death induced by pro-apoptotic molecules later in infection (Yao, L et al., 2010; Yilmaz, O, 2008; Yilmaz, O et al., 2004; Yilmaz, O et al., 2008). P. gingivalis is a successful colonizer of the oral cavity and a major contributor to the etiology of chronic severe periodontitis, has recently been associated with other chronic conditions including the cancers of orodigestive tract (Atanasova, KR et al., 2014, 2015; Olsen, I et al., 2016).
Previous studies have investigated the biological importance of secreted Ndk as an effector for P. gingivalis, to intracellularly survive and replicate in human gingival epithelial cells (GECs) (Spooner, R et al., 2012; Yao, L et al., 2010; Yilmaz, O, 2008; Yilmaz, O et al., 2004; Yilmaz, O et al., 2002; Yilmaz, O et al., 2008). Recent studies have characterized P. gingivalis-Ndk as a putative virulence factor translocated extracellularly of the host epithelial cells in a time-dependent manner during infection (Choi, CH et al., 2013; Spooner, R et al., 2012; Yilmaz, O, 2008; Yilmaz, O et al., 2008). The extracellular release of P. gingivalis-Ndk from host epithelial cell does not occur as a result of compromised host membranes or cell death (Choi, CH et al., 2013;; Yilmaz, O et al., 2008), but rather occurs in a regulated mode using host trafficking machinery via Myosin-9 and leaving host cells through pannexin-1 hemi-channels (Atanasova, K et al., 2016). However, P. gingivalis-Ndk can also be found in the cytoplasm of the infected host cells (Atanasova, K et al., 2016; Choi, CH et al., 2013).
Furthermore, P. gingivalis-Ndk is biologically active with similar GTP and ATP hydrolysis activity as other Ndk homologs in both higher organisms and bacterial species (Choi, CH et al., 2013; Sun, J et al., 2013). The ATP hydrolytic activity of P. gingivalis-Ndk (~0.1 μM s−1) (Yilmaz, O et al., 2008; Choi, CH et al., 2013) is especially important in diminishing the danger signal extracellular ATP(eATP)/P2X7-receptor signaling in primary GECs which generates robust cellular ROS upstream of hypochlorous acid production, a potent eliminator of invading pathogens (Choi, CH et al., 2013; Roberts, JS et al., 2017; Roberts, JS et al., 2015; Yilmaz, O et al., 2008). Moreover P. gingivalis-Ndk is also of importance in the modulation of P2X7-dependent inflammasome activation and the attenuation of secretion of pro-inflammatory cytokine IL-1β as well as pro-inflammatory danger signal HMGB1 in primary GECs (Johnson, L et al., 2015; Olsen, I et al., 2016). However, despite the increasing key importance of Ndk in bacterial survival pathways in host cells, many studies, while observing phenotypic changes attributed to Ndk, do not study specific molecular Ndk-interactors potentially involved in the anti-apoptotic pathways. In our previous studies, we demonstrated that P. gingivalis can inhibit intrinsic apoptosis and activates pro-survival Phosphatidylinositol-4, 5-bisphosphate 3-kinase (PI3K)/AKT signaling in primary GECs. However, the bacterial factor(s) likely involved in this infection induced anti-apoptotic phenotype of the host cells has never been characterized before (Lee, J et al., 2017; Yao, L et al., 2010; Yilmaz, O et al., 2004).
In this study, we investigate the putative interaction between P. gingivalis-Ndk and Heat-Shock-Protein 27 (HSP27) as identified by liquid chromatography-tandem mass spectrometry. HSP27 is a small mammalian cell phosphoprotein (Arrigo, AP, 1998) that regulates apoptosis (Paul, C et al., 2002), lowers ROS levels through increased glutathione levels (Arrigo, AP et al., 2005; Mehlen, P et al., 1997; Preville, X et al., 1999), and stabilizes actin microfilaments (Guay, J et al., 1997) in response to oxidative stress or potent pro-apoptotic drugs such as staurosporine. Recently, there is increasing evidence that point to an important role for HSP27 in tumorigenesis and metastasis in a variety of cancers (Katsogiannou, M et al., 2014; Vidyasagar, A et al., 2012 Cornford, PA et al., 2000; Deyhimi, P et al., 2012; Kapranos, N et al., 2002; Zimmermann, M et al., 2012); therefore making HSP27 a potential therapeutic target (Acunzo, J et al., 2014; Deyhimi, P et al., 2012; Leonardi, R et al., 2002). Moreover, a recent investigation has briefly demonstrated the increase of HSP27 in oral cancer epithelial cells infected with P. gingivalis and its importance in the activation of matrix metalloproteinase 9 (MMP9) in infected cells (Inaba, H et al., 2014). However, HSP27 has not yet been investigated in the context of P. gingivalis colonization or survival in the oral cavity; neither have the molecular mechanisms of HSP27 phosphorylation during infection been defined.
Our results demonstrate a novel kinase function of a bacterial Ndk, specifically P. gingivalis-Ndk, to directly bind and phosphorylate a host protein, HSP27, on specific serine residues. In addition, we illuminate the significance of the Ndk-HSP27 interaction in promoting primary GEC survival during the infection by inhibiting pro-apoptotic Cytochrome C release and Caspase-9 activation. Thus, we demonstrate for the first time the phosphorylation of HSP27 by a bacterial effector - P. gingivalis-Ndk - which confers an anti-apoptotic phenotype to primary GECs. This suggests HSP27 is a critical molecule for P. gingivalis-induced suppression of host cell apoptosis which appears essential for the microorganism’s prolonged intracellular survival in host. Therefore, the findings of this study may translate to identifying targeted strategies to disrupt the establishment of P. gingivalis and other chronic opportunistic pathogens employing Ndk for successful colonization and persistence strategy in human mucosa.
Results
Identification of HSP27 as a putative Ndk interactor by mass spectrometry
Ndks can work both directly or indirectly with molecules in the host cell to facilitate membrane trafficking, manipulate metabolic systems, and affect host survival signaling pathways (Atanasova, K et al., 2016; Atanasova, KR et al., 2014, 2015; Chakrabarty, AM, 1998; Choi, CH et al., 2013; Spooner, R et al., 2012). As a secreted virulence factor from P. gingivalis, Ndk can be present in the host cytoplasm (Atanasova, K et al., 2016; Choi, CH et al., 2013) and carries both ATPase (Choi, CH et al., 2013; Yilmaz, O et al., 2008) and GTPase functions (Supplementary Figure 1). The intracellular function of P. gingivalis-Ndk, however, has not been characterized. Therefore, we examined possible host cytoplasmic interactors from infected primary GEC lysates with recombinant P. gingivalis-Ndk using a GST-pull down assay and performing a liquid-chromatography-tandem-mass-spectrometry analysis of the proteins co-immunoprecipitated with P. gingivalis-Ndk. This analysis revealed Heat shock protein beta-1/HSP27 (IPI00025512.2) as a strong binder to Ndk, with over 95% specificity and over 99.9% threshold in the detection, at a setting of 5 or more matching peptides (Keller, A et al., 2002; Nesvizhskii, AI et al., 2003) (Supplementary Figure 2). HSP27 is an ATP-independent oligomeric phosphoprotein which functions as a negative regulator of intrinsic apoptosis and oxidative stress (Paul, C et al., 2002; Rogalla, T et al., 1999) and thereby promoting cell survival. Therefore, the interaction between Ndk and HSP27 may be critical for P. gingivalis in promoting resistance against intrinsic apoptosis in primary GECs (which has been previously described), which is important for the intracellular life of P. gingivalis in the oral mucosa.
P. gingivalis-Ndk binds directly to HSP27 and increases phosphorylation on activating serine residues 78 and 82
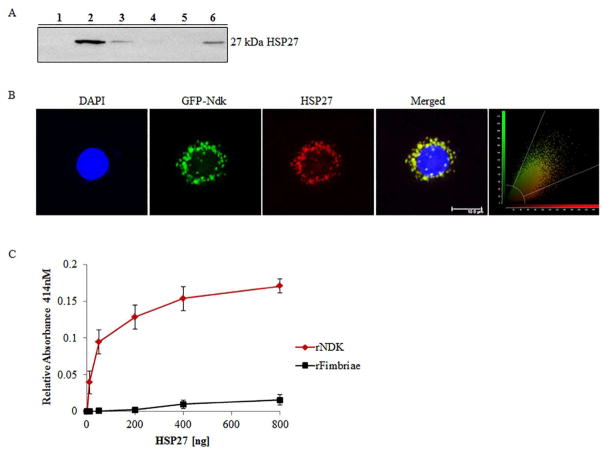
We confirmed the mass spectrometry results by detecting HSP27 specifically in the eluted proteins from the GST-pull down assay by Western blotting (Figure 1A). Further confirmation of the Ndk/HSP27 interaction was assessed using confocal microscopy which demonstrated a high co-localization rate (Manders coefficient of 0.92) of transfected GFP-Ndk with native HSP27 in primary GECs (Figure 1B). Moreover, an ELISA binding assay using purified recombinant (rNdk) and HSP27 showed a concentration-dependent increase in HSP27 binding to Ndk (Figure 1C).
Figure 1. HSP27 binds P. gingivalis Ndk in primary GECs.
(A) Western blot analysis of the presence of HSP27 in the eluent of a GST-pull down of rNdk incubated with primary GEC cytosolic lysate. Lane Designations: 1) Negative control – GST-resin with GEC cell lysate only; 2) GST-resin, P. gingivalis NDK, and GEC cell lysate; 3) Prey flow-through from Lane 1; 4) Prey flow-through from Lane 2; 5) Bait flow-through from Lane 2; 6) Positive control – cell lysate from GECs. (B) Representative confocal micrograph of primary GECs transfected with GFP-Ndk (green) and immunostained for HSP27 (red) with DAPI and representative confocal colocalization analysis scatterplot Zeiss; Bar 10μm. NIH Image J analysis of HSP27 and Ndk co-localization calculated Manders Coefficient as 0.92; n=21. (C) ELISA binding assay of His-tagged recombinant Ndk (rNdk immobilized on a 96-well plate and incubated with varied amounts of HSP27. Absorbance reflective of the amount of HSP27 bound was measured at 414nm. His-tagged recombinant-fimbriae of P. gingivalis were added for comparison of non-specific binding. All the assays were repeated at least in three separate experiments.
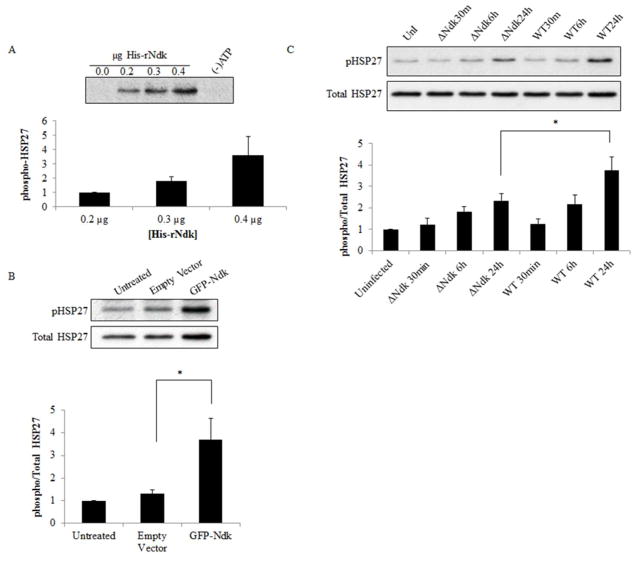
We then examined the biological significance of the Ndk/HSP27 interaction by assessing the phosphorylation state of HSP27 on activating residues serine 78 and 82 (Gusev, NB et al., 2002; Lambert, H et al., 1999) using an in vitro phosphorylation assay. This in vitro reaction demonstrates P. gingivalis-Ndk can directly phosphorylate HSP27 in the presence of ATP and affects HSP27 phosphorylation in a concentration-dependent manner (Figure 2A). Furthermore, overexpression of GFP-Ndk in primary GECs significantly increased phospho-HSP27 as compared to a control empty vector (Figure 2B). In the presence of Wild-type P. gingivalis infection or its isogenic ndk-deficient mutant strain infection, Western blot analysis revealed a gradual significant increase in phospho-HSP27 in primary GECs infected with Wild-type P. gingivalis as compared to the ndk-deficient P. gingivalis in 24 hours (Figure 2C). Slightly increased levels of phosphoHSP27 are still present in ndk-deficient-infected GECs, which is most likely due to the host responding to microbial presence, since HSP27 is a well-characterized cellular stress response protein (Rogalla, T et al., 1999; Takayama, S et al., 2003)(REFs). Taken together, these data show the specific ability of P. gingivalis-Ndk to increase HSP27 phosphorylation on activating residues.
Figure 2. P. gingivalis effector, Ndk, significantly increases HSP27 phosphorylation in primary GECs.
(A) In vitro phosphorylation was conducted with varied amounts of His-rNdk incubated with purified recombinant human HSP27 [0.2μg] and ATP as a substrate (see methods). Negative controls included the reaction without rNdk or without (−) ATP. The phosphorylation result was analyzed via Western blot using phospho Ser78/82 specific antibody for HSP27. For each separate experiment band intensities were analyzed using NIH Image J analysis. n=3; *p<0.05; mean +/ SD. (B) GECs were transfected with Empty vector GFP or GFP-Ndk for 48 hours and analyzed via Western blotting for phospho and Total HSP27. (C) Primary GECs were infected with Wild-type (WT) or ndk-deficient (ΔNdk) P. gingivalis for 30 minutes, 6 hours, or 24 hours at MOI 100. Equal amounts of cell lysates of Uninfected (UnI) and infected GECs were analyzed via Western blotting of phospho-HSP27 and Total HSP27.
P. gingivalis requires HSP27 to block staurosporine-induced cell death via Ndk in primary GECs
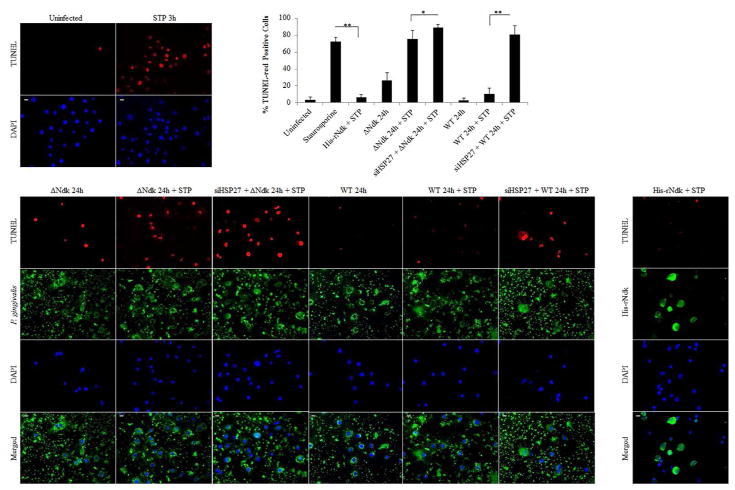
We have previously shown the ability of Wild-type P. gingivalis to inhibit intrinsic apoptosis mediated by a potent pro-apoptotic agent, staurosporine in primary GECs through the time-dependent modulation of Bcl-2 family proteins and the activation of PI3K/AKT pro-survival pathway (Yao, L et al., 2010; Yilmaz, O et al., 2004). However, the importance of HSP27 as an anti-apoptotic molecule during the infection of GECs has not been defined. Therefore, we first assess the potential role of HSP27 in protecting primary GECs against the staurosporine-induced cell death in the presence of infection. We visualized and quantified apoptotic cells using fluorescent microscopy analysis of a TUNEL-TMR red assay which stains nicked DNA. As observed before, Wild-type P. gingivalis infection protected against staurosporine-induced cell death, whereas the ndk-deficient infection failed to show any significant resistance against apoptosis as compared to staurosporine treatment only (Figure 3). On the other hand, down-regulation of HSP27 by siRNA (Supplementary Figure 3) in the presence of staurosporine, however, significantly increased cell death (abolished the protection against apoptosis) during Wild-type infection and further exacerbated cell death in the presence of ndk-deficient P. gingivalis infection (Figure 3A and B). Transfection of the His-tagged recombinant P. gingivalis-Ndk protein into staurosporine-treated primary GECs, however, reversed the effects of staurosporine and thus reduced cell death. Taken together, these results show the key importance of HSP27 as a protective molecule against cell death in primary GECs during infection and P. gingivalis-Ndk plays a critical role for this function of HSP27.
Figure 3. TUNEL analysis of Host Cell Apoptosis in Primary GECs Under Different Conditions.
(A) Representative micrographs of TUNEL stained GECs either transfected with His-rNdk [1μg], infected with Wild-type (WT) or ndk-deficient (ΔNdk) P. gingivalis at MOI 100 for 24 hours and/or treated with pro-apoptotic agent, Staurosporine (STP) [1μM] for 3 hours. Negative controls for non-target siRNA, siHSP27 and the protein transfection reagent only were comparable to untreated (not shown). The assays were repeated at least in three separate experiments. 20x images were taken on Zeiss Axio Imager 1; Bar 10μm. (B) Cell counts of cells undergoing cell-death represented as percent TUNEL-red positive cells. n>100; *p<0.05 and **p<0.001; mean +/ SD.
P. gingivalis-Ndk is vital for reduced Cytochrome C release and Caspase-9 activation during staurosporine-induced cell death
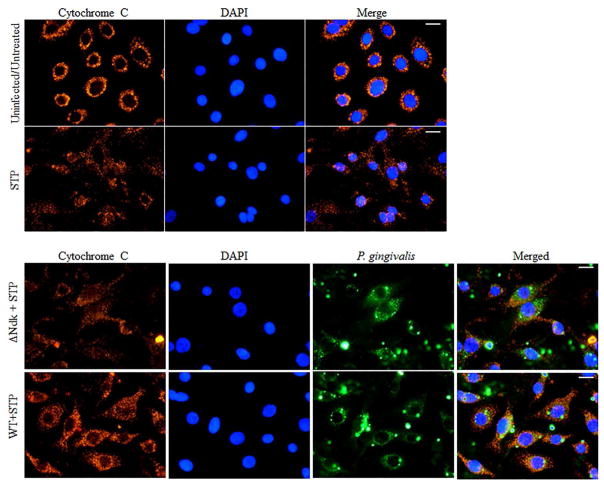
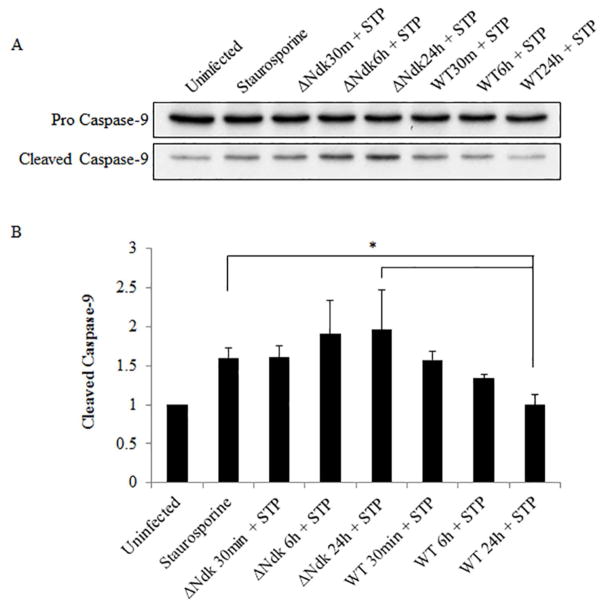
It is well established that HSP27 protects the cell from programmed cell death via the intrinsic apoptosis pathway specifically acting as a negative regulator of Cytochrome C release (Paul, C et al., 2002). Furthermore, we have previously reported the ability of Wild-type P. gingivalis to inhibit staurosporine-induced cell death by abrogating Cytochrome C release and inhibition of mitochondrial membrane depolarization in primary GECs (Yilmaz, O et al., 2004). However, the role of P. gingivalis effector Ndk in the modulation of intrinsic apoptosis has never been investigated previously. Therefore, we analyzed the ability of ndk-deficient or Wild-type P. gingivalis infection to abrogate staurosporine-induced Cytochrome C release using immunofluorescence microscopy. Our results show that ndk-deficient P. gingivalis is unable to maintain Cytochrome C sequestration inside the mitochondria whereas the Wild-type P. gingivalis infected cells displayed intact mitochondria and thus inhibited staurosporine-induced Cytochrome C release (Figure 4). Cytochrome C release activates Procaspase-9 which leads to the formation of an apoptosome (Chinnaiyan, AM, 1999; Elmore, S, 2007). Therefore, we examined the active cleaved form of Caspase-9 in primary GECs infected with Wild-type or ndk-deficient P. gingivalis. Western blot analyses reveal that ndk-deficient P. gingivalis infection significantly increases pro-apoptotic Caspase-9 activation over 24 hours as compared to Wild-type (which is comparable to untreated) (Figure 5). These suggest that inhibition of intrinsic apoptosis during P. gingivalis infection in GECs can occur in a stepwise fashion through its effector, Ndk. Ndk’s induction of HSP27 leads to enhanced blockings of Cytochrome C release from mitochondria and Caspase 9 activation. HSP27, with an ability to interact with a large number of proteins in a sequential manner, has been established to tightly control/inhibit the intrinsic apoptotic pathway via direct binding to Cytochrome C (Paul, C et al., 2002; Takayama, S et al., 2003).
Figure 4. Ndk-deficient mutant strain of P. gingivalis does not inhibit Staurosporine-induced Cytochrome C release in primary GECs.
Uninfected and infected GECs, with either Wild-type (WT) or ndk-deficient (ΔNdk) P. gingivalis at MOI 100, were incubated with pro-apoptotic agent, Staurosporine (STP) [1μM] for 3 hours. Cells were immunostained for Cytochrome C (red) and P. gingivalis (green) and mounted with DAPI. Weaker and largely dispersed red fluorescence indicates release of Cytochrome C from the mitochondria into the cytosol of GECs. ~100 cells were examined for each condition. The assays were repeated at least in three separate experiments. 40x images were taken on Zeiss Axio Imager 1; Bar 10μm.
Figure 5. Active Caspase-9 is increased in primary GECs infected with ndk-deficient P. gingivalis and treated with Staurosporine.
Uninfected and infected GECs at 30 minutes, 6 hours, and 24 hours post infection were treated with 1.0 μM of Staurosporine (STP) for 3 hours. Wild-type P. gingivalis (WT); ndk-deficient P. gingivalis (ΔNdk). (A) Cell lysates were analyzed via Western blot. (B) The band intensities were measured using NIH ImageJ software and presented as a ratio of pro- to cleaved caspase-9. Data is represented as mean +/− SD; n=4; *p < 0.05.
Discussion
The regulation of phosphorylation has critical importance in determining the function of a protein and has significant importance in enabling the host cell to quickly respond to stimuli. The function and specific protein interaction of HSP27 is regulated by its phosphorylation state which promotes the formation of small oligomers that can interact with target molecules such as pro-apoptotic Cytochrome C (Katsogiannou, M et al., 2014; Paul, C et al., 2002). This leads to inhibition of apoptosis. The unphosphorylated state of HSP27 forms larger oligomers but can also modulate key cellular functions (Hayes, D et al., 2009; Lentze, N et al., 2004). For example, in the unphosphorylated state HSP27 can cap actin filaments, thus inhibiting polymerization, (Benndorf, R et al., 1994) whereas phosphorylated HSP27 promotes polymerization (Guay, J et al., 1997; Huot, J et al., 1996). Another example of dual roles regulated by phosphorylation can be seen in the modulation of glutathione levels by phosphorylated HSP27 (Arrigo, AP et al., 2005; Rogalla, T et al., 1999; Preville, X et al., 1999).
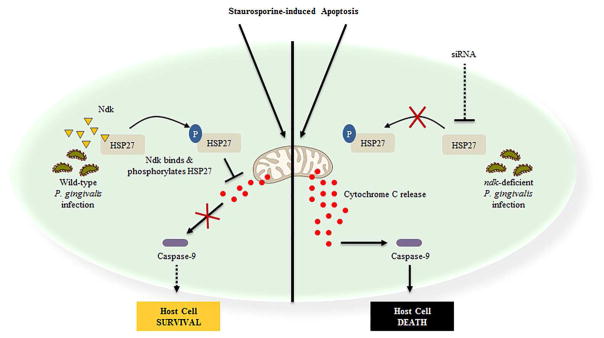
P. gingivalis, a facultatively intracellular bacterium, can replicate and survive in GECs without being overtly detrimental to the host and modulate the epithelial cell response as an effective strategy for host immune evasion and persistence (Yilmaz, O, 2008). Our findings summarized in Figure 6, show the novel ability of P. gingivalis-Ndk to bind and directly phosphorylate HSP27 on serine residues 78 and 82. The in vitro phosphorylation assay and use of recombinant Ndk constructs confirm that the observed increase in HSP27 phosphorylation is significantly due to P. gingivalis-Ndk. In this way, P. gingivalis-Ndk serves as a phospho-regulator of HSP27 in primary GECs during the infection. Increased phospho-HSP27 contributes to protection of the infected cell from Cytochrome C/Caspase-9 dependent cell death, which are hallmark characteristics of the intrinsic apoptosis pathway (Elmore, S, 2007). This mechanism of action of HSP27 has been well defined in literature (Elmore, S, 2007; Paul, C et al., 2002) albeit it has not been characterized in human GECs. Therefore, we show for the first time that HSP27 is largely critical in conferring apoptotic resistance to GECs during P. gingivalis infection.
Figure 6. Proposed model of the P. gingivalis Ndk in subversion of GEC cell death through HSP27 phosphorylation.
Previously studies have shown P. gingivalis-Ndk is released from bacterial cells, secreted intracellularly, and can be present in the cytoplasm of primary GECs (Atanasova, K et al., 2016; Choi, CH et al., 2013; Olsen, I et al., 2016; Yilmaz, O et al., 2008). The ability of P. gingivalis to subvert intrinsic apoptosis was also characterized phenotypically by Cytochrome C release and Caspase 9/Caspase 3 inhibitions (Yao, L et al., 2010, Yilmaz, O et al., 2004); however, a molecular bacterial mechanism has not been characterized. P. gingivalis-Ndk binds and directly phosphorylates HSP27 at serine residues 78/82 leading to subsequent blocking of Cytochrome C release and inhibition of Caspase-9-dependent cell death (intrinsic apoptosis). Conversely, in the absence of Ndk and/or depletion of HSP27 during infection, there is a significant increase in Caspase-9-dependent cell death in human primary GECs. Thus, via the action of Ndk effector, P. gingivalis can exercise intricate control over and subvert cell death pathways, specifically apoptosis, thereby allowing its organismal intracellular replication and survival to continue.
Ndks are present in a variety of different organismal species and operate as a multifunctional enzyme in the both prokaryotic and eukaryotic cells (Chopra, P et al., 2004; Dar, HH et al., 2011; Spooner, R et al., 2012). Typically, Ndk enzymes mediate phosphorylation of histidine residues (Adam, K et al., 2017; Attwood, PV et al., 2015) and, to our knowledge, only one study, using human form of Ndk (designated as Nme), has reported phosphotransferase activity leading to phosphorylation on serine and threonine residues (Engel, M et al., 1995). As we have previously reported, P. gingivalis-Ndk, which can be secreted from the microorganism intracellularly during the host infection and translocated extracellularly in a time-dependent mode, can deplete eATP thereby hindering P2X7-receptor signaling and subsequent cellular ROS generation (Choi, CH et al., 2013; Yilmaz, O, 2008; Yilmaz, O et al., 2008). The substantial effects of Ndk and HSP27 interaction are markedly detected early as 6 hours post-infection and maximized at 24 hours post-infection. The largest increase in phosphorylation of HSP27, which we show is directly attributed to Ndk, is observed at 24 hours post-infection causing inhibition of cell death. Therefore, over the course of infection, P. gingivalis may use its effector Ndk in a multifaceted approach to sustain a safe environment for its intracellular life and survival in the primary GECs. It is tempting to suggest that other opportunistic pathogens which possess Ndk (Spooner, R et al., 2012; Yu, H et al., 2017) may utilize this effector molecule for modulation of cellular signaling that critically involved in pathogen persistence in the host (Atanasova, K et al., 2016; Choi, CH et al., 2013; Kim, YJ, Lee, JH, et al., 2014; Kim, YJ, Paek, SH, et al., 2014).
Therefore, this study provides a novel framework to build on the potential effects of the phospho-regulatory role of Ndk on HSP27 during the infection of GECs. Furthermore, P. gingivalis has recently been shown to increase HSP27 in oral cancer epithelial cells functioning upstream of MMP9 important for degrading the ECM and promoting invasion of cancer cells (Inaba, H et al., 2014). HSP27 is overexpressed in many cancer cell types leading to poor prognoses (Arrigo, AP et al., 2005; Cornford, PA et al., 2000; Guay, J et al., 1997; Katsogiannou, M et al., 2014; Mehlen, P et al., 1997; Vidyasagar, A et al., 2012). Hence, it has been recently investigated as a viable therapeutic target in cancer therapies using anti-sense oligonucleotides and/or pharmacological inhibitors to diminish HSP27 (Baylot, V et al., 2011; Hsu, HS et al., 2011; Vidyasagar, A et al., 2012).
Therefore, given the role of HSP27 for the protection against chemically induced -intrinsic- apoptosis of primary GECs in the presence of P. gingivalis, HSP27 may be an essential target to promote clearance of P. gingivalis in the oral mucosa. Further, the rising association and proposed causative roles of microbes with cancer (Atanasova, KR et al., 2015), also suggests that the insight gained in this study of the Ndk-HSP27 interaction, could point to future targeted therapeutic strategies against other chronic diseases (e.g. cancer) recently associated with P. gingivalis infection. Interestingly, Human nm23/nucleoside diphosphate kinase (Nme) has also been investigated for its involvement in the metastatic potential of tumor cells, where it can be found in elevated levels secreted from tumor cells both in vitro and in vivo (Huwer, H et al., 1997; Niitsu, N, Honma, Y, et al., 2003; Niitsu, N, Nakamine, H, et al., 2003; Okabe-Kado, J et al., 1992). The shared conserved homology of bacterial and human Ndks (Schneider, B et al., 2001; Yilmaz, O et al., 2008; Yu, H et al., 2017) compels the examination of different Ndk species and whether they may function similarly in the context of tumorigenesis and metastasis.
In summary, our study demonstrates a novel kinase function for P. gingivalis-Ndk in phosphorylating host cell HSP27 on specific serine residues, not previously described for any bacterial effector and/or Ndk family of proteins. Furthermore, our findings suggest the specific role of HSP27 for host cell survival in the context of P. gingivalis-mediated resistance against intrinsic apoptosis in primary GECs. Ultimately, the bacterial control of the host cell death pathways via its effector molecule, Ndk is essential for optimal intracellular life and establishment of P. gingivalis infection in the oral mucosa. The knowledge gained from this study may have significant implications for future therapeutic strategies for chronic microbial diseases and other conditions where Ndks are a virulence factor.
Experimental Procedures
Bacterial and Primary Cell Culture
Primary human gingival epithelial cells (GECs) were obtained and cultured as described previously (Oda, D et al., 1990; Yilmaz, O et al., 2002; Yilmaz, O et al., 2008). Briefly, gingival tissue was collected from patients who were selected anonymously and randomly from those presenting for tooth crown lengthening or impacted third molar extraction under the approved guidance of the University of Florida Health Science Center Institutional Review Board (IRB, human subjects assurance number FWA 00005790). No patient information was collected and informed consent was obtained by all subjects. Cells were cultured in serum-free keratinocyte growth medium (KGM, Lonza) at 37 °C in 5% CO2.
P. gingivalis ATCC 33277 strain and its isogenic ndk-deficient mutant strain were cultured to mid-log phase in Trypticase soy broth (TSB) supplemented with yeast extract (1 μg/ml), menadione (1 μg/ml) and hemin (5 μg/ml), at 37 °C under anaerobic conditions and harvested as previously described (Yilmaz, O et al., 2008). Erythromycin (10 mg/ml) was added to the media as a selective agent for the growth of the mutant strain. The bacterial pellets were re-suspended in Dulbecco’s phosphate-buffered saline (PBS) containing calcium and magnesium (HyClone) and bacteria were quantified using a Klett-Summerson photometer. GECs were infected with the bacteria at a multiplicity of infection (MOI) of 100 in all experiments.
GTPase Assay
His-tagged recombinant P. gingivalis-Ndk (1.4 μg) (GenScript) was incubated at 25°C with 1 mM GTP for 30 min and the liberated inorganic phosphate (Pi) was measured using the GTPase colorimetric assay kit (Innova Biosciences; Babraham, Cambridge, UK) according to manufacturer instructions. BSA (10 μg) was used as a negative control. To overcome high background due to the presence of Pi in the initial buffers that rNdk or BSA were stored in, we pre-treated with PiBind resin (Innova Biosciences) overnight at 4°C with gentle mixing followed by centrifugation at low speed (100 ×g for 1 min). The supernatant was checked for Pi contamination according to the kit manual (Innova Biosciences). The amount of Pi produced was determined from a standard curve expressing GTPase activity as μmoL of Pi generated per moL of rNdk per minute.
GST Pull-down assay and Liquid chromatography-tandem MASS-Spectrometry (LC-MS/MS) analysis
To identify putative cellular binding partners for P. gingivalis-Ndk, we conducted glutathione S-transferase (GST)-mediated pull-down assay. Primary GECs were infected with P. gingivalis and after one hour of infection, cells were washed with DPBS and then fractionated using a mitochondria/cytosol fractionation kit (BioVision). The N-terminal GST-tagged recombinant P. gingivalis-Ndk (100μg; GenScript) was immobilized onto 25 μl of glutathione resin (ThermoFisher Scientific) at 4 °C for one hour. The immobilized Ndk was then incubated with 100 μg of the collected GEC proteins from the fractionated lysates (either mitochondrial of cytosolic) at 4 °C overnight. In order to eliminate non-specific binding (false positive) to the immobilized glutathione, we incubated 100 μg of GEC proteins with 25 μl of glutathione resin in the absence of recombinant Ndk and eluted the bound proteins to serve as a negative control. Eluted proteins from the GST-pull down assay were visualized using 12% Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and subsequent Coomassie staining. Stained protein band(s) found only in the recombinant Ndk pull-down as compared to the negative control, were submitted to LC-MS/MS analysis, performed at the Interdisciplinary Center for Biotechnology Research of the University of Florida (Atanasova, K et al., 2016). Briefly, the MS/MS samples were analyzed using Mascot software (Matrix Science, London, UK; version 2.4.0) and Scaffold (version Scaffold-4.0, Proteome Software Inc., Portland, OR) was used to validate the MS/MS peptide and protein identifications. In the Scaffold software, results representing 95% or higher specificity and over 99.9% threshold of detection, at a setting of no less than 5 matching peptides for each detected protein, was considered significant.
Mass spectrometry confirmation through Western blotting
The GST-pull down was performed as described above and the eluted immune-precipitated proteins were run on an SDS-PAGE polyacrylamide gel and probed for HSP27 as described in Western Blotting methods.
Confocal microscopy
GECs were seeded directly on glass coverslips in four-well cell culture plates and were transfected with a plasmid, pEGFP-C1 (gift from Dr. Fredrick Southwick) transformed with a P. gingivalis ATCC 33277 NDK insert (gene PGN_1337) at 90% cell confluence using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. pEGFP-C1 vector, not containing an insert (empty vector) was used as control. After 48 hours of transfection, the cells were fixed with 10% neutral buffered formalin for 30 minutes and then permeabilized with 0.01% Triton X-100 for 30 minutes. The fixed cells were incubated with mouse monoclonal anti-HSP27 antibody (Santa Cruz) at a dilution of 1:100 at room temperature for 1 h. Following the incubation with the primary antibody, the cells were washed three times with PBS and subsequently incubated with secondary antibody, Alexa Fluor568 goat anti-mouse at a dilution of 1:500 (Invitrogen), at room temperature for 1 h. After antibody staining, the cells were washed with PBS and mounted on glass slides with mounting media containing 4′, 6-diamidino-2-phenylindole (DAPI) and visualized using confocal microscopy (Zeiss). The co-localization coefficient of P. gingivalis-Ndk and HSP27 was measured using NIH ImageJ with the JACoP plugin as described previously (Bolte, S et al., 2006). The co-localization rate was measured based on Manders coefficient ranging from 0 to 1, where zero indicates non-overlapping and a value of 1 reflects a 100% overlap of the two analyzed images (Choi, CH et al., 2011).
Measurement of HSP27 and P. gingivalis-Ndk by ELISA Binding Assay
96-well enzyme-linked immunosorbent assay (ELISA) plates were coated with 300 ng His-tagged P. gingivalis-Ndk (GenScript) per well in carbonate/bicarbonate buffer (Sigma) at 4°C overnight. The wells were blocked with PBS containing 0.05% Tween 20 and 0.5% BSA for 1 h at room temperature. Varied amounts (0, 10, 50, 20, 400, and 800 ng) of purified recombinant HSP27 (Santa Cruz) were added to the immobilized Ndk and incubated for 2 h at room temperature. After the ELISA plates were washed, bound HSP27 was detected with anti-HSP27 mouse antibody (Cell Signaling), followed by HRP-conjugated goat anti-mouse antibody (Cell Signaling). After washing, colorimetric substrate, 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) in a citric acid-sodium phosphate buffer (Sigma) was added to the wells and incubated for 1 h at room temperature. HRP activity was read at an absorbance of 414 nm using a Synergy MX plate reader (BioTek Instruments). Plates were coated with His-tagged recombinant P. gingivalis 33277 fimbriae (Yilmaz, O et al., 2002) as a control comparison for non-specific binding.
Western Blot Analyses
Western blot analysis was used to evaluate the level of phosphorylated HSP27 in GECs during P. gingivalis ATCC 33277 strain or ndk-deficient mutant strain infection: 30 minutes, 6 hours, and 24 hours. Uninfected and infected GECs were collected at the same stage of growth to mitigate any effects of cellular aging on the protein composition and HSP27 phosphorylation. Total proteins were extracted from the infected GECs using RIPA lysis buffer (Cell Signaling) plus protease and phosphatase inhibitors: 1mM PMSF; 0.1mM TLCK; 1mM NaF; 2mM N-ethylmaleimide; 1 mM sodium orthovanadate; and aprotinin (10 μg/ml). The protein concentration of each sample was measured using a standard Bradford assay (BioRad) and equal amounts of protein (20 μg) were loaded onto a 12% SDS polyacrylamide gel. After gel electrophoresis, the proteins were transferred onto a nitrocellulose membrane using wet-transfer system and the membrane was blocked in Tris-buffered saline with 0.1% Tween 20 (TBST) containing 5% nonfat dry milk. The membrane was immunoblotted overnight at 4°C with anti-phospho-HSP27 rabbit polyclonal antibody (R&D Systems) at a dilution of 1:1000 in TBST. Horseradish peroxidase conjugated anti-rabbit antibody (Invitrogen) was used for secondary labelling at 1:1,000 in TBST for 1 h at room temperature. The membrane was re-probed with a Total HSP27 mouse antibody (Santa Cruz) and β-tubulin mouse antibody (Invitrogen) at 1:1,000 in TBST. A horseradish peroxidase conjugated anti-mouse (Invitrogen) was used to visualize both anti-total HSP27 and β-tubulin. Protein bands were visualized by enhanced chemiluminescence (ECL, GE Healthcare). Phospho- and Total HSP27 was also detected by Western blot in primary GEC lysates after transfection with either control plasmid, pEGFP-C1, or a plasmid expressing Ndk, pEGFP-C1-Ndk, at 90% cell confluence using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol after 48 hours of transfection.
Western blot was also used to analyze the expression of pro and cleaved caspase-9. GECs were infected with P. gingivalis ATCC 33277 or its isogenic ndk-deficient mutant for 30 minutes, 6 hours, and 24 hours and were treated with 1.0 μM of staurosporine (Sigma) for 5 hours before collection. Total proteins were extracted from the GECs using RIPA buffer plus protease and phosphatase inhibitors as described before. Equal amounts of total protein from the samples were separated by SDS-PAGE and transferred on to a nitrocellulose membrane. Pro- and cleaved caspase-9 were detected using overnight incubation at 4°C with anti-caspase-9 rabbit antibody (Cell Signaling) at a dilution of 1:1000 in TBST. Secondary antibody, horseradish peroxidase conjugated anti-rabbit (Cell Signaling), was used at 1:1000 in TBST, and protein bands were visualized using ECL. Protein band intensities were examined using NIH ImageJ.
In vitro phosphorylation
In order to confirm the role of P. gingivalis-Ndk in phosphorylation of HSP27, we performed in vitro phosphorylation of purified recombinant HSP27 (Santa Cruz) with recombinant His-tagged P. gingivalis-Ndk (GenScript) 50 mM Tris, pH 7.5; 2.5 mM MgCl2; and 0.3 mM ATP in a total volume of 20 μl at 30°C for 30 minutes. Each reaction contained 0.2 μg of recombinant HSP27. Three reactions contained recombinant P. gingivalis-Ndk at different quantities: 0.2 μg, 0.3 μg, and 0.4 μg. The phosphorylation reaction was terminated by the addition of 4x Laemmli sample buffer (Bio-Rad). The proteins were separated by SDS-PAGE and HSP27 phosphorylation was detected as described in the methods section titled western blotting. The intensities of protein bands were analyzed using NIH ImageJ software.
Silencing of HSP27
Primary GECs were seeded in 6-well plates and transfected with SignalSilence HSP27 siRNA II [100nM] (Cell Signaling) or Control siRNA [100nM] (Life Technologies) using Lipofectamine RNAiMax Protocol (Invitrogen) for 48 hours. Cell lysates were collected and equal amounts of protein were collected for Western Bot analysis as described. HSP27 was detected using an anti-mouse HSP27 antibody (Santa Cruz) followed by β-tubulin mouse antibody as a loading control (Invitrogen) at 1:1000 in TBST. A horseradish peroxidase conjugated anti-mouse (Invitrogen) was used to visualize both anti-total HSP27 and β-tubulin. Protein band intensities were examined using NIH ImageJ.
TUNEL Assay
Primary GECs were seeded in 4-well plates on glass inserts and transfected with SignalSilence HSP27 siRNA II [100nM] or Control siRNA [100nM]. After 24 hours of transfection, GECs were infected with either Wild-type P. gingivalis 33277 strain or isogenic ndk-deficient mutant strain for 24 hours. Staurosporine [1μM] (Sigma) was added for 3 hours before collection (21 hours post-infection). To examine P. gingivalis infection and nicked DNA, the infected and Staurosporine treated GECs were fixed with 10% neutral buffered formalin for 30 minutes and permeabilized for 15 min in PBS containing 0.1% Triton X-100. The permeabilized cells were incubated with a polyclonal rabbit anti- P. gingivalis 33277 antibody at 1:1000 dilution in PBS containing 0.1% Tween 20 for 1 h. After washing with PBS-Tween 20, cells were immunostained with Alexa Fluor 488 goat anti-rabbit immunoglobulin G (Invitrogen) at 1:1000 dilution at ambient temperature for 1 h. The cells were then incubated with TUNEL-TMR Red (Sigma/Roche) reaction mix according to manufacturer’s instructions for 1 hour at 37°C. The immunostained cells were then washed with PBS and mounted on glass slides using Vectashield mounting media with DAPI, and examined using wide-field fluorescence microscope (Zeiss Axio Imager A1). The images were captured using a cooled charge-coupled device camera controlled by QCAPTURE software (Qimaging). Quantification was then determined using cell counts for TUNEL-positive cells; n>100.
His-rNdk Protein Transfection
Primary GECs were seeded in 4-well plates on glass inserts and transfected with 1 μg of His-rNdk (GenScript) using Pierce Protein Transfection Reagent (ThermoScientific) according to the manufacturer’s protocol. Briefly, the His-rNdk was diluted in PBS and mixed with 2.5 μL/well of the dried transfection reagent for 5 minutes. The mixture was then added to the GECs for 21 hours and then Staurosporine [1μM] (Sigma) subsequently added for 3 hours. His-rNdk was detected using anti-rabbit His-tag antibody (GenScript) 1:50 and Alexa Fluor 488 goat anti-rabbit immunoglobulin G (Invitrogen) 1:1000. The remaining steps for TUNEL and Immunofluorescence were as described above. TUNEL-TMR red positive cells are represented as percent positive cells out of the total number of cells assessed; n>100.
Immunofluorescence
To examine Cytochrome C distribution, GECs were infected with P. gingivalis ATCC 33277 strain or its ndk-deficient mutant strain and incubated for 21 hours. At 21 hours post infection, the infected GECs were treated for 3 hours with staurosporine (1.0 μM) (Sigma) and permeabilized with digitonin (50 μg/ml) (Sigma) in PBS supplemented with KCl (100 mM) for 5 minutes on ice (Yilmaz, O et al., 2004). The cells were then immediately fixed in 10% neutral buffered formalin for 30 minutes and permeabilized for 30 minutes in PBS containing 0.01% Triton X-100. The permeabilized cells were incubated with mouse anti-Cytochrome C monoclonal antibody (BD Pharmingen) at 1:100 dilution and rabbit anti-P. gingivalis 33277 polyclonal antibody at 1:1000 in PBS containing 0.1% Tween 20 for 1 h. After washing with PBS-Tween 20, cells were immunostained with Alexa Fluor568 goat anti-mouse (Invitrogen) at 1:500 dilution and Alexa Fluor488 goat anti-rabbit (Invitrogen) at 1:1,000 dilution at room temperature for 1 h. Digitonin treatment of GECs prior to fixation allowed selective permeabilization of the plasma membrane and diffusion of cytosolic Cytochrome C out of the cells, so that the GECs with high cytoplasmic Cytochrome C levels have less fluorescence than the cells with intact mitochondria (Yilmaz, O et al., 2004). The immunostained cells were mounted on glass slides using Vectashield mounting media with DAPI, and examined using wide-field fluorescence microscope (Zeiss Axio Imager A1). The images were captured using a cooled charge-coupled device camera controlled by QCAPTURE software (Qimaging).
Supplementary Material
Acknowledgments
This work was supported by funding from the NIDCR grants R01 DE016593, T90 DE021990, T32 DE017551 and F31DE026065. The authors declare no conflict of interest and no competing financial interests. This work does not represent the official views of the NIH and is solely the responsibility of the authors.
References
- Acunzo J, Andrieu C, Baylot V, So A, Rocchi P. Hsp27 as a therapeutic target in cancers. Curr Drug Targets. 2014;15(4):423–431. doi: 10.2174/13894501113146660230. [DOI] [PubMed] [Google Scholar]
- Adam K, Hunter T. Histidine kinases and the missing phosphoproteome from prokaryotes to eukaryotes. Lab Invest. 2017 doi: 10.1038/labinvest.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo AP. Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. Biol Chem. 1998;379(1):19–26. [PubMed] [Google Scholar]
- Arrigo AP, Virot S, Chaufour S, Firdaus W, Kretz-Remy C, Diaz-Latoud C. Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxid Redox Signal. 2005;7(3–4):414–422. doi: 10.1089/ars.2005.7.414. [DOI] [PubMed] [Google Scholar]
- Atanasova K, Lee J, Roberts J, Lee K, Ojcius DM, Yilmaz O. Nucleoside-Diphosphate-Kinase of P. gingivalis is Secreted from Epithelial Cells In the Absence of a Leader Sequence Through a Pannexin-1 Interactome. Sci Rep. 2016;6:37643. doi: 10.1038/srep37643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanasova KR, Yilmaz O. Looking in the Porphyromonas gingivalis cabinet of curiosities: the microbium, the host and cancer association. Molecular Oral Microbiology. 2014;29(2):55–66. doi: 10.1111/omi.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanasova KR, Yilmaz O. Prelude to oral microbes and chronic diseases: past, present and future. Microbes and Infection. 2015;17(7):473–483. doi: 10.1016/j.micinf.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwood PV, Wieland T. Nucleoside diphosphate kinase as protein histidine kinase. Naunyn Schmiedebergs Arch Pharmacol. 2015;388(2):153–160. doi: 10.1007/s00210-014-1003-3. [DOI] [PubMed] [Google Scholar]
- Baylot V, Andrieu C, Katsogiannou M, Taieb D, Garcia S, Giusiano S, … Rocchi P. OGX-427 inhibits tumor progression and enhances gemcitabine chemotherapy in pancreatic cancer. Cell Death Dis. 2011;2:e221. doi: 10.1038/cddis.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. Journal of Biological Chemistry. 1994;269(32):20780–20784. [PubMed] [Google Scholar]
- Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224(Pt 3):213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- Chakrabarty AM. Nucleoside diphosphate kinase: role in bacterial growth, virulence, cell signalling and polysaccharide synthesis. Mol Microbiol. 1998;28(5):875–882. doi: 10.1046/j.1365-2958.1998.00846.x. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan AM. The apoptosome: heart and soul of the cell death machine. Neoplasia. 1999;1(1):5–15. doi: 10.1038/sj.neo.7900003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CH, DeGuzman JV, Lamont RJ, Yilmaz O. Genetic Transformation of an Obligate Anaerobe, P. gingivalis for FMN-Green Fluorescent Protein Expression in Studying Host-Microbe Interaction. PLoS One. 2011;6(4) doi: 10.1371/journal.pone.0018499. ARTN e18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CH, Spooner R, DeGuzman J, Koutouzis T, Ojcius DM, Yilmaz O. Porphyromonas gingivalis-nucleoside-diphosphate-kinase inhibits ATP-induced reactive-oxygen-species via P2X7 receptor/NADPH-oxidase signalling and contributes to persistence. Cellular Microbiology. 2013;15(6):961–976. doi: 10.1111/cmi.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra P, Koduri H, Singh R, Koul A, Ghildiyal M, Sharma K, … Singh Y. Nucleoside diphosphate kinase of Mycobacterium tuberculosis acts as GTPase-activating protein for Rho-GTPases. FEBS Lett. 2004;571(1–3):212–216. doi: 10.1016/j.febslet.2004.06.073. [DOI] [PubMed] [Google Scholar]
- Cornford PA, Dodson AR, Parsons KF, Desmond AD, Woolfenden A, Fordham M, … Foster CS. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000;60(24):7099–7105. [PubMed] [Google Scholar]
- Dar HH, Prasad D, Varshney GC, Chakraborti PK. Secretory nucleoside diphosphate kinases from both intra- and extracellular pathogenic bacteria are functionally indistinguishable. Microbiology. 2011;157(Pt 11):3024–3035. doi: 10.1099/mic.0.049221-0. [DOI] [PubMed] [Google Scholar]
- Deyhimi P, Azmoudeh F. HSP27 and HSP70 expression in squamous cell carcinoma: An immunohistochemical study. Dent Res J (Isfahan) 2012;9(2):162–166. doi: 10.4103/1735-3327.95230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel M, Veron M, Theisinger B, Lacombe ML, Seib T, Dooley S, Welter C. A novel serine/threonine-specific protein phosphotransferase activity of Nm23/nucleoside-diphosphate kinase. Eur J Biochem. 1995;234(1):200–207. doi: 10.1111/j.1432-1033.1995.200_c.x. [DOI] [PubMed] [Google Scholar]
- Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110(Pt 3):357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- Gusev NB, Bogatcheva NV, Marston SB. Structure and properties of small heat shock proteins (sHsp) and their interaction with cytoskeleton proteins. Biochemistry (Mosc) 2002;67(5):511–519. doi: 10.1023/a:1015549725819. [DOI] [PubMed] [Google Scholar]
- Hayes D, Napoli V, Mazurkie A, Stafford WF, Graceffa P. Phosphorylation dependence of hsp27 multimeric size and molecular chaperone function. Journal of Biological Chemistry. 2009;284(28):18801–18807. doi: 10.1074/jbc.M109.011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HS, Lin JH, Huang WC, Hsu TW, Su K, Chiou SH, … Hung SC. Chemoresistance of lung cancer stemlike cells depends on activation of Hsp27. Cancer. 2011;117(7):1516–1528. doi: 10.1002/cncr.25599. [DOI] [PubMed] [Google Scholar]
- Huot J, Houle F, Spitz DR, Landry J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996;56(2):273–279. [PubMed] [Google Scholar]
- Huwer H, Kalweit G, Engel M, Welter C, Dooley S, Gams E. Expression of the candidate tumor suppressor gene nm23 in the bronchial system of patients with squamous cell lung cancer. Eur J Cardiothorac Surg. 1997;11(2):206–209. doi: 10.1016/s1010-7940(97)86704-8. [DOI] [PubMed] [Google Scholar]
- Inaba H, Sugita H, Kuboniwa M, Iwai S, Hamada M, Noda T, … Amano A. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cellular Microbiology. 2014;16(1):131–145. doi: 10.1111/cmi.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Atanasova KR, Bui PQ, Lee J, Hung SC, Yilmaz O, Ojcius DM. Porphyromonas gingivalis attenuates ATP-mediated inflammasome activation and HMGB1 release through expression of a nucleoside-diphosphate kinase. Microbes and Infection. 2015;17(5):369–377. doi: 10.1016/j.micinf.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapranos N, Kominea A, Konstantinopoulos PA, Savva S, Artelaris S, Vandoros G, … Papavassiliou AG. Expression of the 27-kDa heat shock protein (HSP27) in gastric carcinomas and adjacent normal, metaplastic, and dysplastic gastric mucosa, and its prognostic significance. J Cancer Res Clin Oncol. 2002;128(8):426–432. doi: 10.1007/s00432-002-0357-y. [DOI] [PubMed] [Google Scholar]
- Katsogiannou M, Andrieu C, Rocchi P. Heat shock protein 27 phosphorylation state is associated with cancer progression. Front Genet. 2014;5:346. doi: 10.3389/fgene.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith KE, Hynes DW, Sholdice JE, Valvano MA. Delayed association of the NADPH oxidase complex with macrophage vacuoles containing the opportunistic pathogen Burkholderia cenocepacia. Microbiology. 2009;155(Pt 4):1004–1015. doi: 10.1099/mic.0.026781-0. [DOI] [PubMed] [Google Scholar]
- Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74(20):5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Lee JH, Lee Y, Jia J, Paek SH, Kim HB, … Ha UH. Nucleoside diphosphate kinase and flagellin from Pseudomonas aeruginosa induce interleukin 1 expression via the Akt/NF-kappaB signaling pathways. Infection and Immunity. 2014;82(8):3252–3260. doi: 10.1128/IAI.02007-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Paek SH, Jin S, Park BS, Ha UH. A novel Pseudomonas aeruginosa-derived effector cooperates with flagella to mediate the upregulation of interleukin 8 in human epithelial cells. Microb Pathog. 2014;66:24–28. doi: 10.1016/j.micpath.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Kumar P, Verma A, Saini AK, Chopra P, Chakraborti PK, Singh Y, Chowdhury S. Nucleoside diphosphate kinase from Mycobacterium tuberculosis cleaves single strand DNA within the human c-myc promoter in an enzyme-catalyzed reaction. Nucleic Acids Res. 2005;33(8):2707–2714. doi: 10.1093/nar/gki568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert H, Charette SJ, Bernier AF, Guimond A, Landry J. HSP27 multimerization mediated by phosphorylation-sensitive intermolecular interactions at the amino terminus. Journal of Biological Chemistry. 1999;274(14):9378–9385. doi: 10.1074/jbc.274.14.9378. [DOI] [PubMed] [Google Scholar]
- Lee J, Roberts JS, Atanasova K, Chowdhury N, Han K, Yilmaz O. Human Primary Epithelial Cells Acquire An Epithelial-Mesenchymal-Transition Phenotype during Long-term Infection by The Oral Opportunistic Pathogen, Porphyromonas gingivalis. Frontiers in Cellular and Infection Microbiology. 2017 doi: 10.3389/fcimb.2017.00493. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentze N, Narberhaus F. Detection of oligomerisation and substrate recognition sites of small heat shock proteins by peptide arrays. Biochem Biophys Res Commun. 2004;325(2):401–407. doi: 10.1016/j.bbrc.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Leonardi R, Pannone G, Magro G, Kudo Y, Takata T, Lo Muzio L. Differential expression of heat shock protein 27 in normal oral mucosa, oral epithelial dysplasia and squamous cell carcinoma. Oncol Rep. 2002;9(2):261–266. [PubMed] [Google Scholar]
- Mehlen P, Hickey E, Weber LA, Arrigo AP. Large unphosphorylated aggregates as the active form of hsp27 which controls intracellular reactive oxygen species and glutathione levels and generates a protection against TNFalpha in NIH-3T3-ras cells. Biochem Biophys Res Commun. 1997;241(1):187–192. doi: 10.1006/bbrc.1997.7635. [DOI] [PubMed] [Google Scholar]
- Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75(17):4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- Niitsu N, Honma Y, Iijima K, Takagi T, Higashihara M, Sawada U, Okabe-Kado J. Clinical significance of nm23-H1 proteins expressed on cell surface in non-Hodgkin’s lymphoma. Leukemia. 2003;17(1):196–202. doi: 10.1038/sj.leu.2402699. [DOI] [PubMed] [Google Scholar]
- Niitsu N, Nakamine H, Okamoto M, Akamatsu H, Honma Y, Higashihara M … Adult Lymphoma Treatment Study Group A. Expression of nm23-H1 is associated with poor prognosis in peripheral T-cell lymphoma. Br J Haematol. 2003;123(4):621–630. doi: 10.1046/j.1365-2141.2003.04668.x. [DOI] [PubMed] [Google Scholar]
- Oda D, Watson E. Human oral epithelial cell culture I. Improved conditions for reproducible culture in serum-free medium. In Vitro Cell Dev Biol. 1990;26(6):589–595. doi: 10.1007/BF02624208. [DOI] [PubMed] [Google Scholar]
- Okabe-Kado J, Kasukabe T, Honma Y, Hayashi M, Henzel WJ, Hozumi M. Identity of a differentiation inhibiting factor for mouse myeloid leukemia cells with NM23/nucleoside diphosphate kinase. Biochem Biophys Res Commun. 1992;182(3):987–994. doi: 10.1016/0006-291x(92)91829-f. [DOI] [PubMed] [Google Scholar]
- Olsen I, Yilmaz O. Modulation of inflammasome activity by Porphyromonas gingivalis in periodontitis and associated systemic diseases. J Oral Microbiol. 2016;8:30385. doi: 10.3402/jom.v8.30385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP. Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol. 2002;22(3):816–834. doi: 10.1128/MCB.22.3.816-834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preville X, Salvemini F, Giraud S, Chaufour S, Paul C, Stepien G, … Arrigo AP. Mammalian small stress proteins protect against oxidative stress through their ability to increase glucose-6-phosphate dehydrogenase activity and by maintaining optimal cellular detoxifying machinery. Experimental Cell Research. 1999;247(1):61–78. doi: 10.1006/excr.1998.4347. [DOI] [PubMed] [Google Scholar]
- Ray NB, Mathews CK. Nucleoside diphosphokinase: a functional link between intermediary metabolism and nucleic acid synthesis. Curr Top Cell Regul. 1992;33:343–357. doi: 10.1016/b978-0-12-152833-1.50025-3. [DOI] [PubMed] [Google Scholar]
- Roberts JS, Atanasova KR, Lee J, Diamond G, Deguzman J, Hee Choi C, Yilmaz O. Opportunistic Pathogen Porphyromonas gingivalis Modulates Danger Signal ATP-Mediated Antibacterial NOX2 Pathways in Primary Epithelial Cells. Front Cell Infect Microbiol. 2017;7:291. doi: 10.3389/fcimb.2017.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JS, Yilmaz Dangerous Liaisons: Caspase-11 and Reactive Oxygen Species Crosstalk in Pathogen Elimination. International Journal of Molecular Sciences. 2015;16(10):23337–23354. doi: 10.3390/ijms161023337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, … Gaestel M. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. Journal of Biological Chemistry. 1999;274(27):18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- Schneider B, Babolat M, Xu YW, Janin J, Veron M, Deville-Bonne D. Mechanism of phosphoryl transfer by nucleoside diphosphate kinase pH dependence and role of the active site Lys16 and Tyr56 residues. Eur J Biochem. 2001;268(7):1964–1971. doi: 10.1046/j.1432-1327.2001.02070.x. [DOI] [PubMed] [Google Scholar]
- Spooner R, Yilmaz O. Nucleoside-diphosphate-kinase: a pleiotropic effector in microbial colonization under interdisciplinary characterization. Microbes and Infection. 2012;14(3):228–237. doi: 10.1016/j.micinf.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Singh V, Lau A, Stokes RW, Obregon-Henao A, Orme IM, … Hmama Z. Mycobacterium tuberculosis nucleoside diphosphate kinase inactivates small GTPases leading to evasion of innate immunity. Plos Pathogens. 2013;9(7):e1003499. doi: 10.1371/journal.ppat.1003499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S, Reed JC, Homma S. Heat-shock proteins as regulators of apoptosis. Oncogene. 2003;22(56):9041–9047. doi: 10.1038/sj.onc.1207114. [DOI] [PubMed] [Google Scholar]
- Vidyasagar A, Wilson NA, Djamali A. Heat shock protein 27 (HSP27): biomarker of disease and therapeutic target. Fibrogenesis Tissue Repair. 2012;5(1):7. doi: 10.1186/1755-1536-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Jermanus C, Barbetta B, Choi C, Verbeke P, Ojcius DM, Yilmaz O. Porphyromonas gingivalis infection sequesters pro-apoptotic Bad through Akt in primary gingival epithelial cells. Molecular Oral Microbiology. 2010;25(2):89–101. doi: 10.1111/j.2041-1014.2010.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O. The chronicles of Porphyromonas gingivalis: the microbium, the human oral epithelium and their interplay. Microbiology. 2008;154(Pt 10):2897–2903. doi: 10.1099/mic.0.2008/021220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O, Jungas T, Verbeke P, Ojcius DM. Activation of the phosphatidylinositol 3-kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infection and Immunity. 2004;72(7):3743–3751. doi: 10.1128/IAI.72.7.3743-3751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O, Watanabe K, Lamont RJ. Involvement of integrins in fimbriae-mediated binding and invasion by Porphyromonas gingivalis. Cellular Microbiology. 2002;4(5):305–314. doi: 10.1046/j.1462-5822.2002.00192.x. [DOI] [PubMed] [Google Scholar]
- Yilmaz O, Yao L, Maeda K, Rose TM, Lewis EL, Duman M, … Ojcius DM. ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X(7)-mediated host-cell apoptosis. Cellular Microbiology. 2008;10(4):863–875. doi: 10.1111/j.1462-5822.2007.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Rao X, Zhang K. Nucleoside diphosphate kinase (Ndk): A pleiotropic effector manipulating bacterial virulence and adaptive responses. Microbiol Res. 2017;205:125–134. doi: 10.1016/j.micres.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Zimmermann M, Nickl S, Lambers C, Hacker S, Mitterbauer A, Hoetzenecker K, … Ankersmit HJ. Discrimination of clinical stages in non-small cell lung cancer patients by serum HSP27 and HSP70: a multi-institutional case-control study. Clin Chim Acta. 2012;413(13–14):1115–1120. doi: 10.1016/j.cca.2012.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.